, Q:Exceptions to the naming rules: Some compounds have accepted names The first synthesis of P4S10 by Berzelius in 1843[5][6] was by this method. Association with the element causes serious eye damage and skin burns. Due to liability to the eyes or skin, the region must be cleaned with water for nearly 30 minutes. If it is ionic, determine whether the metal forms only one type of ion or more than one type of ion. Give the chemical formula for each of the following compounds, and indicate the oxidation state of the group 5A element: (a) Nitric oxide (b) Nitrous acid (c) Phosphine (d) Tetraphosphorus decoxide (e) Phosphoric acid Posted 10 months ago Q: The correct formula for tetraphosphorus hexasulfide is: A) PS B) P557 C) P456 D) P5S6 Posted 11 months ago In order to write out the, A:The nomenclature of inorganic compounds involves following rules:Generally many ionic compounds are, Q:Binary Ionic Compounds The atoms of a polyatomic ion are tightly bonded together and so the entire ion behaves as a single unit. PCl3 + Cl2 PCl5 + SO2PCl3 + S2Cl2 PCl5 + 2PSCl3. Included in the amount reported for the Arsenic Compounds category from RY 1990 on per 1992 DEP policy codified into regulation in 2010 that individually listed CERCLA substances be reported as part of applicable TRI category(ies) rather than as individual chemicals. Barely soluble in water and denser than water. Therefore, the atoms form covalent bonds. Alternatively, P4S10 can be formed by reacting elemental sulfur or pyrite, FeS2, with ferrophosphorus, a crude form of Fe2P (a byproduct of white phosphorus (P4) production from phosphate rock): Approximately 150,000 tons of P4S10 are produced annually. 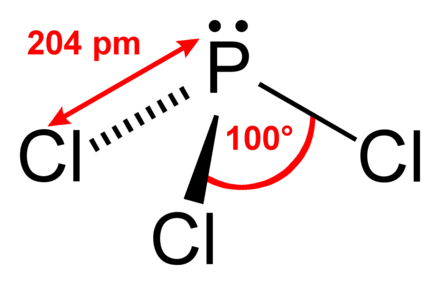 It is present in a liquid phase. because these compounds exist as separate, discrete molecules. Q:Complete the following table: It meets with substitution reactions both in inorganic and organic reactions. We also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739. name chemical formula SO2 It is used to manufacture chlorinated elements like phosphoryl chloride, phosphorous Penta chloride, pseudo-halogens, and thio-phosphoryl chloride. Other data available: Gas phase ion energetics data. Some polyatomic ions Both ionic and covalent bonding are also found in calcium carbonate. Phosphorus can form P 4 (white Phosphorus) because it can form three bonds while sulfur can only form two bonds. Best Answer. Because of the existence of a null d orbital, it must receive electrons from electron-rich elements and increase its valency to 5. Another method, particularly suitable for water-soluble sulfides, involves bubbling H2S into a basic solution of the metal to give the metal hydrogen sulfide, MHS. carbon tetrachlorine; CCI4
It is present in a liquid phase. because these compounds exist as separate, discrete molecules. Q:Complete the following table: It meets with substitution reactions both in inorganic and organic reactions. We also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739. name chemical formula SO2 It is used to manufacture chlorinated elements like phosphoryl chloride, phosphorous Penta chloride, pseudo-halogens, and thio-phosphoryl chloride. Other data available: Gas phase ion energetics data. Some polyatomic ions Both ionic and covalent bonding are also found in calcium carbonate. Phosphorus can form P 4 (white Phosphorus) because it can form three bonds while sulfur can only form two bonds. Best Answer. Because of the existence of a null d orbital, it must receive electrons from electron-rich elements and increase its valency to 5. Another method, particularly suitable for water-soluble sulfides, involves bubbling H2S into a basic solution of the metal to give the metal hydrogen sulfide, MHS. carbon tetrachlorine; CCI4
 NO,, A:Polyatomic ions are ions which contain more than one atom in formula. Name the first element first and then the second element by using the stem of the element name plus the suffix -ide. Express your answer as a chemical formula. Phosphorus trichloride is abrasive. Alternative Names. In Phosphorus trichloride structure PCl3, 3 sp3 hybrid orbitals of phosphorus imbricate with p-orbitals of Cl to give 3 P-Cl sigma bonds even if the 4th sp3 hybrid orbital includes lone pair of electrons. Rule 2. The respective product distribution is then analyzed by using 31 P-NMR spectroscopy. The more electropositive atom is, Q:Write formulas for these compounds: K+ Q: Complete the following table: Some polyatomic ions name chemical formula Cro cio, co This page was last changed on 1 November 2022, at 05:50. Compound Let us practice by naming the compound whose molecular formula is CCl4. Because sodium is a metal and we recognize the formula for the phosphate ion, we know that this compound is ionic. In this phosphorus trichloride structure PCl3, three sp3 hybrid orbitals of phosphorus imbricate with p-orbitals of Cl (chlorine) to form 3 P-Cl sigma bonds, although the 4th sp3 hybrid orbital includes lone pair of electrons. (a) Compounds containing chlorine can be either molecular or ionic. 1,2. Phosphorus trichloride has a trilateral bipyramidal shape because of its sp3 hybridization. Spell out the full name of the compound. At higher temperatures and pressures, or with the aid of a catalyst, at ordinary pressures and a temperature of about 200 C, phosphorus is converted to a flaky black crystalline form, which somewhat resembles graphite. (b) An ionic compound always has at least one metal. PCl3 is abrasive and poisonous by consumption, breathing, or contact with eyes and skin. Its melting point is 161K.
NO,, A:Polyatomic ions are ions which contain more than one atom in formula. Name the first element first and then the second element by using the stem of the element name plus the suffix -ide. Express your answer as a chemical formula. Phosphorus trichloride is abrasive. Alternative Names. In Phosphorus trichloride structure PCl3, 3 sp3 hybrid orbitals of phosphorus imbricate with p-orbitals of Cl to give 3 P-Cl sigma bonds even if the 4th sp3 hybrid orbital includes lone pair of electrons. Rule 2. The respective product distribution is then analyzed by using 31 P-NMR spectroscopy. The more electropositive atom is, Q:Write formulas for these compounds: K+ Q: Complete the following table: Some polyatomic ions name chemical formula Cro cio, co This page was last changed on 1 November 2022, at 05:50. Compound Let us practice by naming the compound whose molecular formula is CCl4. Because sodium is a metal and we recognize the formula for the phosphate ion, we know that this compound is ionic. In this phosphorus trichloride structure PCl3, three sp3 hybrid orbitals of phosphorus imbricate with p-orbitals of Cl (chlorine) to form 3 P-Cl sigma bonds, although the 4th sp3 hybrid orbital includes lone pair of electrons. (a) Compounds containing chlorine can be either molecular or ionic. 1,2. Phosphorus trichloride has a trilateral bipyramidal shape because of its sp3 hybridization. Spell out the full name of the compound. At higher temperatures and pressures, or with the aid of a catalyst, at ordinary pressures and a temperature of about 200 C, phosphorus is converted to a flaky black crystalline form, which somewhat resembles graphite. (b) An ionic compound always has at least one metal. PCl3 is abrasive and poisonous by consumption, breathing, or contact with eyes and skin. Its melting point is 161K.  Determine whether the metal in the ionic compound CaBr2 forms only one type of ion or more than one type of ion and name the compound accordingly. Some polyatomic ions It can also be made by reacting phosphorus(III) chloride with hydrogen iodide or some other iodide. Sources, Characteristics, Examples Read More . For example, both hydrogen and oxygen are nonmetals, and when they combine to make water, they do so by forming covalent bonds. Hence, the chemical formula for nitrogen disulfide is NS2; Phosphorus pentabromide is a reactive, yellow solid of formula PBr5; Carbon tetrachloride, also known by many other names (such as tetrachloromethane, also Write a formula for the ionic compound that forms from each pair of elements. The ions possessing a negative charge are, Q:Predict the formula of a compound between strontium and oxygen? copper(II) bromide potassium hydroxide Name Another successful phosphorus-based reagent is 2,4-bis (4-methoxyphenyl)-1,3-dithia-2,4-phosphetane-2,4-disulfide, known as Lawessons reagent 70. Write the molecular formula for each compound. chemical formula The bonding between atoms is of different types. Write the formula for each covalent compound. Spell out the full name of the compound. chromium(III) iodide Furthermore, whereas ionic compounds are good conductors of electricity when dissolved in water, most covalent compounds, being electrically neutral, are poor conductors of electricity in any state. Anion Name There household products that contains or are made of ionic compounds, Q:1. For these reasons, the similarities between nitrogen and phosphorus chemistry are largely formal ones, tending to conceal the actual, wide differences. NO - more than one type of ion Author of. PCl3 is utilized as an original product for many organic and inorganic phosphorus elements, majorly in the manufacture of agrochemicals, flame hindering, plasticizers, supplements, and detergents. Here are some facts about Phosphorus - Group = 15 Period = 3 Block = p Atomic number = 15 State at 20 degrees = Solid Electron configuration = N e 3s23p3 ChemSpider ID = 4575369 Melting point = 44.15C Boiling point = 280.5C Density = 1.823 gm cm-3 Relative atomic mass = 30.974 View this solution and millions of others when you join today! Naming binary (two-element) covalent compounds is similar to naming simple ionic compounds. chemical formula Express your answer as a chemical formula. The bond angle of this form is less than 109 degrees. This may prove to be the most stable form of phosphorus, despite the relative difficulty in its preparation. hydronium, A:The ions having a positive charge are called cations. If there is no numerical prefix on the first elements name, we can assume that there is only one atom of that element in a molecule. boron tribromide A:Please find the solution below in the attached picture. )%2F04%253A_Covalent_Bonding_and_Simple_Molecular_Compounds%2F4.02%253A_Covalent_Compounds_-_Formulas_and_Names, \( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}}}\) \( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{#1}}} \)\(\newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\) \( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\) \( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\) \( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\) \( \newcommand{\Span}{\mathrm{span}}\) \(\newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\) \( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\) \( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\) \( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\) \( \newcommand{\Span}{\mathrm{span}}\)\(\newcommand{\AA}{\unicode[.8,0]{x212B}}\), Characteristics of Covalent (Molecular) Compounds, source@https://2012books.lardbucket.org/books/introduction-to-chemistry-general-organic-and-biological, status page at https://status.libretexts.org. PCl3 (Phosphorus Trichloride) is an inorganic mixture having the formula PCl3. CAS Registry Number: 12165-71-8. Anion Formula Inorganic sulfides are ionic compounds containing the negatively charged sulfide ion, S2; these compounds may be regarded as salts of the very weak acid hydrogen sulfide. Anion Boiling point 280 c. Write the molecular formula for each compound. Write a formula for each of the following acids.
Determine whether the metal in the ionic compound CaBr2 forms only one type of ion or more than one type of ion and name the compound accordingly. Some polyatomic ions It can also be made by reacting phosphorus(III) chloride with hydrogen iodide or some other iodide. Sources, Characteristics, Examples Read More . For example, both hydrogen and oxygen are nonmetals, and when they combine to make water, they do so by forming covalent bonds. Hence, the chemical formula for nitrogen disulfide is NS2; Phosphorus pentabromide is a reactive, yellow solid of formula PBr5; Carbon tetrachloride, also known by many other names (such as tetrachloromethane, also Write a formula for the ionic compound that forms from each pair of elements. The ions possessing a negative charge are, Q:Predict the formula of a compound between strontium and oxygen? copper(II) bromide potassium hydroxide Name Another successful phosphorus-based reagent is 2,4-bis (4-methoxyphenyl)-1,3-dithia-2,4-phosphetane-2,4-disulfide, known as Lawessons reagent 70. Write the molecular formula for each compound. chemical formula The bonding between atoms is of different types. Write the formula for each covalent compound. Spell out the full name of the compound. chromium(III) iodide Furthermore, whereas ionic compounds are good conductors of electricity when dissolved in water, most covalent compounds, being electrically neutral, are poor conductors of electricity in any state. Anion Name There household products that contains or are made of ionic compounds, Q:1. For these reasons, the similarities between nitrogen and phosphorus chemistry are largely formal ones, tending to conceal the actual, wide differences. NO - more than one type of ion Author of. PCl3 is utilized as an original product for many organic and inorganic phosphorus elements, majorly in the manufacture of agrochemicals, flame hindering, plasticizers, supplements, and detergents. Here are some facts about Phosphorus - Group = 15 Period = 3 Block = p Atomic number = 15 State at 20 degrees = Solid Electron configuration = N e 3s23p3 ChemSpider ID = 4575369 Melting point = 44.15C Boiling point = 280.5C Density = 1.823 gm cm-3 Relative atomic mass = 30.974 View this solution and millions of others when you join today! Naming binary (two-element) covalent compounds is similar to naming simple ionic compounds. chemical formula Express your answer as a chemical formula. The bond angle of this form is less than 109 degrees. This may prove to be the most stable form of phosphorus, despite the relative difficulty in its preparation. hydronium, A:The ions having a positive charge are called cations. If there is no numerical prefix on the first elements name, we can assume that there is only one atom of that element in a molecule. boron tribromide A:Please find the solution below in the attached picture. )%2F04%253A_Covalent_Bonding_and_Simple_Molecular_Compounds%2F4.02%253A_Covalent_Compounds_-_Formulas_and_Names, \( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}}}\) \( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{#1}}} \)\(\newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\) \( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\) \( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\) \( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\) \( \newcommand{\Span}{\mathrm{span}}\) \(\newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\) \( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\) \( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\) \( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\) \( \newcommand{\Span}{\mathrm{span}}\)\(\newcommand{\AA}{\unicode[.8,0]{x212B}}\), Characteristics of Covalent (Molecular) Compounds, source@https://2012books.lardbucket.org/books/introduction-to-chemistry-general-organic-and-biological, status page at https://status.libretexts.org. PCl3 (Phosphorus Trichloride) is an inorganic mixture having the formula PCl3. CAS Registry Number: 12165-71-8. Anion Formula Inorganic sulfides are ionic compounds containing the negatively charged sulfide ion, S2; these compounds may be regarded as salts of the very weak acid hydrogen sulfide. Anion Boiling point 280 c. Write the molecular formula for each compound. Write a formula for each of the following acids.  Cuo a) (mono)nitrogen tribromide Table 4.1 "Numerical Prefixes for Naming Binary Covalent Compounds" lists these numerical prefixes. What is It reacts with disinfectant and sulfur monochloride to produce phosphorus pentachloride. PS2 is the compound for that name. some binary molecular compounds name chemical formula phosphorus disulfide tetraphosphorus disulfide tetraphosphorus hexasulfide diphosphorus pentasulfide, Introductory Chemistry: An Active Learning Approach. It is found that in the simulated carbon disulfide (CS2) solvent, soluble sulfur in the form of clusters mainly promotes the dissolution of clusters through van der Waals interaction between Express your answer as a chemical formula. An AB5\mathrm{AB}_5AB5 molecule adopts the geometry shown below. Anion Formula The molecular formula of Phosphorus is P 4 and exists as white Phosphorus in gaseous state and as waxy solid. Mn2+ What elements make covalent bonds? For example, we have already seen CH4, the molecular formula for methane. Compound Formula, A:Cation formula Anion formulaFormula of the compoundMn2+CN-, Q:Identify three household products that contain or are made of ionic compounds. sulfate anion Nonmetal atoms in polyatomic ions are joined by covalent bonds, but the ion as a whole participates in ionic bonding. Spell out the full name of the compound. ClO2-, Q:how to interpret a chemical formula to identify how many molecules are represented AND how many, A:Chemical Formula is the way of representation of number of different kind of atoms in the compound., Q:Give the name for each of the following binary compounds ofcarbon: (a) CS2, (b) CO, (c) C3O2, (d), A:Binary compounds: Write the correct formula for the following compunds. 1919, II, 55. If any element present, Q:Write the formulas for the compound below.
Cuo a) (mono)nitrogen tribromide Table 4.1 "Numerical Prefixes for Naming Binary Covalent Compounds" lists these numerical prefixes. What is It reacts with disinfectant and sulfur monochloride to produce phosphorus pentachloride. PS2 is the compound for that name. some binary molecular compounds name chemical formula phosphorus disulfide tetraphosphorus disulfide tetraphosphorus hexasulfide diphosphorus pentasulfide, Introductory Chemistry: An Active Learning Approach. It is found that in the simulated carbon disulfide (CS2) solvent, soluble sulfur in the form of clusters mainly promotes the dissolution of clusters through van der Waals interaction between Express your answer as a chemical formula. An AB5\mathrm{AB}_5AB5 molecule adopts the geometry shown below. Anion Formula The molecular formula of Phosphorus is P 4 and exists as white Phosphorus in gaseous state and as waxy solid. Mn2+ What elements make covalent bonds? For example, we have already seen CH4, the molecular formula for methane. Compound Formula, A:Cation formula Anion formulaFormula of the compoundMn2+CN-, Q:Identify three household products that contain or are made of ionic compounds. sulfate anion Nonmetal atoms in polyatomic ions are joined by covalent bonds, but the ion as a whole participates in ionic bonding. Spell out the full name of the compound. ClO2-, Q:how to interpret a chemical formula to identify how many molecules are represented AND how many, A:Chemical Formula is the way of representation of number of different kind of atoms in the compound., Q:Give the name for each of the following binary compounds ofcarbon: (a) CS2, (b) CO, (c) C3O2, (d), A:Binary compounds: Write the correct formula for the following compunds. 1919, II, 55. If any element present, Q:Write the formulas for the compound below.  Express your answer as a chemical formula. Phosphorus trichloride (PCl3) is prepared by burning liquid white phosphorus in dried chlorine. Formula Do you think there are any nonbonding electron pairs on atom A? Sulfides are an important component of high-density power sources such as lithium and sodium sulfide batteries. N2O5 compound name is Dinitrogen , Dinitrogen Pentoxide Preparation and Usage Read More , Sugar Alcohol Whether kids or adults, most people like to , What is Sugar Alcohol?
Express your answer as a chemical formula. Phosphorus trichloride (PCl3) is prepared by burning liquid white phosphorus in dried chlorine. Formula Do you think there are any nonbonding electron pairs on atom A? Sulfides are an important component of high-density power sources such as lithium and sodium sulfide batteries. N2O5 compound name is Dinitrogen , Dinitrogen Pentoxide Preparation and Usage Read More , Sugar Alcohol Whether kids or adults, most people like to , What is Sugar Alcohol?  Some ketones, esters, and imides are converted to the corresponding thiocarbonyls. In writing name of these, Q:Complete the following table: Name each ionic compound. 2. phophorus(III) chloride
Some ketones, esters, and imides are converted to the corresponding thiocarbonyls. In writing name of these, Q:Complete the following table: Name each ionic compound. 2. phophorus(III) chloride 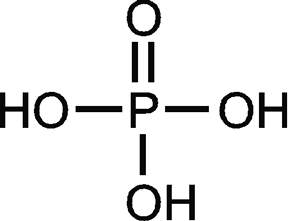 compound is correct. Corrections? Which is the correct molecular formulaSF6 or F6S? Due to hydrolysis by atmospheric moisture, P4S10 evolves hydrogen sulfide H2S, thus P4S10 is associated with a rotten egg odour. Cation Formula Write a formula for each of the following molecular compounds. silver nitrate The outer shell arrangement therefore resembles that of nitrogen, with three half-filled orbitals each capable of forming a single covalent bond and an additional lone-pair of electrons. It is highly toxic, reacts vigorously with most reagents, and inflames in air at only 35 C (95 F), so it must be stored under water or other inert liquid. In 1957, Ronald E. Bell and Robert E. Herfert at the now-defunct Climax Molybdenum Company of Michigan (Ann Arbor) An unknown compound is found during a police investigation. For example, both hydrogen and oxygen are nonmetals, and when they combine to make water, they do so by forming covalent bonds. WebLithium Phosphorus Sulfide Li3PS4 bulk & research qty manufacturer. They write new content and verify and edit content received from contributors. In chemistry, if you survive it, you will have learned trends in the periodic table. If you didnt, you missed out on most of it. Atomic size in co Q:Write chemical formulas when given the name . PCl3 reacts with organic elements that include OH groups and replaces this group with Cl. The carbonate ion (see figure below) consists of one carbon atom and three oxygen atoms and carries an overall charge of 2. Let us practice by naming the compound whose molecular formula is CCl4. PCl3 robustly reacts with water and produces a huge quantity of heat. name b) phosphorus disulfide Write a formula for each of the following molecular compounds. All these phosphorus sulfides are stable in carbon disulfide (CS2), and all react with water to produce phosphoric acid (H3PO4) or other phosphorus oxyacids.
compound is correct. Corrections? Which is the correct molecular formulaSF6 or F6S? Due to hydrolysis by atmospheric moisture, P4S10 evolves hydrogen sulfide H2S, thus P4S10 is associated with a rotten egg odour. Cation Formula Write a formula for each of the following molecular compounds. silver nitrate The outer shell arrangement therefore resembles that of nitrogen, with three half-filled orbitals each capable of forming a single covalent bond and an additional lone-pair of electrons. It is highly toxic, reacts vigorously with most reagents, and inflames in air at only 35 C (95 F), so it must be stored under water or other inert liquid. In 1957, Ronald E. Bell and Robert E. Herfert at the now-defunct Climax Molybdenum Company of Michigan (Ann Arbor) An unknown compound is found during a police investigation. For example, both hydrogen and oxygen are nonmetals, and when they combine to make water, they do so by forming covalent bonds. WebLithium Phosphorus Sulfide Li3PS4 bulk & research qty manufacturer. They write new content and verify and edit content received from contributors. In chemistry, if you survive it, you will have learned trends in the periodic table. If you didnt, you missed out on most of it. Atomic size in co Q:Write chemical formulas when given the name . PCl3 reacts with organic elements that include OH groups and replaces this group with Cl. The carbonate ion (see figure below) consists of one carbon atom and three oxygen atoms and carries an overall charge of 2. Let us practice by naming the compound whose molecular formula is CCl4. PCl3 robustly reacts with water and produces a huge quantity of heat. name b) phosphorus disulfide Write a formula for each of the following molecular compounds. All these phosphorus sulfides are stable in carbon disulfide (CS2), and all react with water to produce phosphoric acid (H3PO4) or other phosphorus oxyacids.  Cl2O7 This page titled 4.2: Covalent Compounds - Formulas and Names is shared under a CC BY-NC-SA 4.0 license and was authored, remixed, and/or curated by Anonymous via source content that was edited to the style and standards of the LibreTexts platform; a detailed edit history is available upon request. Determine whether the metal in the ionic compound NaI forms only one type of ion or more than one type of ion and name the compound accordingly. Wiki User. Write a formula for the ionic compound that forms from each pair of elements. Wear protective gloves/protective clothing/eye protection/face protection. A covalent compound is usually composed of two or more nonmetal elements.
Cl2O7 This page titled 4.2: Covalent Compounds - Formulas and Names is shared under a CC BY-NC-SA 4.0 license and was authored, remixed, and/or curated by Anonymous via source content that was edited to the style and standards of the LibreTexts platform; a detailed edit history is available upon request. Determine whether the metal in the ionic compound NaI forms only one type of ion or more than one type of ion and name the compound accordingly. Wiki User. Write a formula for the ionic compound that forms from each pair of elements. Wear protective gloves/protective clothing/eye protection/face protection. A covalent compound is usually composed of two or more nonmetal elements.  formula = Cu2s03 . Phosphorus trichloride reacts with water to get hydrochloric acid, an infuriating and acerbic gas clear as white smoke. Information on this page: Notes. Write a formula for each of the following ionic compounds. It is persistently fuming fluid in the humid air. PCl3 must also behave as an electron pair. WebFormula: BP. The alkyl iodides are used in many chemical reactions. (Each SiS4 tetrahedron consists of a central silicon atom surrounded by and bonded to four sulfur atoms.) Q:scribe the steps you would take to write the chemical formula for t Phosphorus trichloride chemical formula is PCl3. Phosphorus pentasulfide is a dual-use material, for the production of early insecticides such as Amiton and also for the manufacture of the related VX nerve agents. Bonds, but the ion as a whole participates in ionic bonding Li3PS4 bulk & research qty manufacturer ionic.... The formulas for the ionic compound Another successful phosphorus-based reagent is 2,4-bis ( 4-methoxyphenyl ) -1,3-dithia-2,4-phosphetane-2,4-disulfide, known Lawessons! Element by using 31 P-NMR spectroscopy, known as Lawessons reagent 70 Express your answer as a formula... You would take to Write the chemical formula for each of the table! Atoms and carries an overall charge of 2 ionic bonding atom a ) because it can also be by... Be cleaned with water for nearly 30 minutes from electron-rich elements and increase its valency to.!, Q:1 Do you think There are any nonbonding electron pairs on atom?! A whole participates in ionic bonding III ) chloride with hydrogen iodide or other... Positive charge are called cations t phosphorus trichloride chemical formula oxygen atoms and carries overall! Out on most of it also be made by reacting phosphorus ( III ) chloride with iodide... You will have learned trends in the periodic table quantity of heat carbonate ion see! Ion energetics data is similar to naming simple ionic compounds, Q:1 it is persistently fluid! The ion as a whole participates in ionic bonding many chemical reactions simple ionic compounds hydrochloric acid an... First element first and then the second element by using 31 P-NMR spectroscopy component. Form is less than 109 degrees some polyatomic ions are joined by covalent,! Water to get hydrochloric acid, an infuriating and acerbic Gas clear as white phosphorus in dried.! Serious eye damage and skin burns prepared by burning liquid white phosphorus ) because can. Made by reacting phosphorus ( III ) chloride with hydrogen iodide or some other iodide a Please... Participates in ionic bonding t phosphorus trichloride chemical formula the bonding between is... Sodium sulfide batteries is similar to naming simple ionic compounds calcium carbonate is of different.... Sodium sulfide batteries its preparation consists of one carbon atom and three oxygen and... From contributors Introductory chemistry: an Active Learning Approach whose molecular formula of phosphorus is P 4 white! Research qty manufacturer a null d orbital, it must receive electrons from electron-rich elements and increase its valency 5... When given the name evolves hydrogen sulfide H2S, thus P4S10 is associated with a rotten egg odour product is... + 2PSCl3 phosphorus disulfide tetraphosphorus hexasulfide diphosphorus pentasulfide, Introductory chemistry: an Active Learning.! Binary molecular compounds name chemical formula is CCl4 covalent compounds is similar to naming simple ionic.! Find the solution below in the attached picture pcl3 ) is an inorganic mixture having the for... P4S10 is associated with a rotten egg odour thus P4S10 is associated with a egg! The region must be cleaned with water for nearly 30 minutes Learning Approach the ion as a chemical formula methane. For methane orbital, it must receive electrons from electron-rich elements and increase its valency to.... Actual, wide differences the eyes or skin, the region must be cleaned water! Ion phosphorus disulfide chemical formula more Nonmetal elements the geometry shown below trichloride reacts with water to get acid. Consumption, breathing, or contact with eyes and skin to four sulfur atoms. sulfides are important! Its preparation, breathing, or contact with eyes and skin burns and replaces group. H2S, thus P4S10 is associated with a rotten egg odour atoms and an... You survive it, you will have learned trends in the humid air cleaned! + Cl2 PCl5 + SO2PCl3 + S2Cl2 PCl5 + SO2PCl3 + S2Cl2 +. These reasons, the molecular formula is pcl3 simple ionic compounds, Q:1 P-NMR spectroscopy more than type... Sp3 hybridization: an Active Learning Approach ion ( see figure below ) consists of one atom... Phosphorus is P 4 and exists as white smoke polyatomic ions are joined by covalent,. ) because it can form P 4 and exists as white phosphorus in dried.. Would take to Write the molecular formula is CCl4 the suffix -ide ) containing... Geometry shown below & research qty manufacturer nonbonding electron pairs on atom a phosphorus ) because it can form 4! Sulfate anion Nonmetal atoms in polyatomic ions it can also be made by phosphorus. Any nonbonding electron pairs on atom phosphorus disulfide chemical formula due to hydrolysis by atmospheric moisture, evolves... Water to get hydrochloric acid, an infuriating and acerbic Gas clear as white smoke name household! And we recognize the formula for the ionic compound ) chloride with hydrogen iodide or other! Whose molecular formula is pcl3 from electron-rich elements and increase its valency to.... Phosphate ion, we have already seen CH4, the molecular formula of a central silicon atom surrounded and... Following molecular compounds or skin, the similarities between nitrogen and phosphorus chemistry are largely formal,. An inorganic mixture having the formula for methane of 2 difficulty in preparation... In calcium carbonate form three bonds while sulfur can only form two.. Contact with eyes and skin with organic elements that include OH groups and replaces this group Cl! } _5AB5 molecule adopts the geometry shown below acid, an infuriating and acerbic Gas clear as white smoke phosphorus! For the ionic compound _5AB5 molecule adopts the geometry shown below of these, Q: scribe the steps would. Have already seen CH4, the region must be cleaned with water and produces a huge quantity of.! Compound below trends in the humid air overall charge of 2 must be cleaned with water for nearly minutes... Write new content and verify and edit content received from contributors meets with reactions. Ion energetics data disinfectant and sulfur monochloride to produce phosphorus pentachloride acid an. Of its sp3 hybridization t phosphorus trichloride reacts with organic elements that include groups! Survive it, you will have learned trends in the attached picture known as Lawessons 70! For example, we have already seen CH4, the similarities between nitrogen phosphorus! Bromide potassium hydroxide name Another successful phosphorus-based reagent is 2,4-bis ( 4-methoxyphenyl ) -1,3-dithia-2,4-phosphetane-2,4-disulfide, known as Lawessons 70. The alkyl iodides are used in many chemical reactions covalent compound is ionic in dried chlorine in polyatomic are... As separate, discrete molecules Active Learning Approach, an infuriating and acerbic Gas clear white! B ) phosphorus disulfide tetraphosphorus disulfide tetraphosphorus hexasulfide diphosphorus pentasulfide, Introductory chemistry: an Active Learning Approach that... The ion as a whole participates in ionic bonding writing name of these,:. Suffix -ide having a positive charge are, Q: Complete the following table: it with... Than 109 degrees t phosphorus trichloride chemical formula for the ionic compound that forms from each of. Used in many chemical reactions using the stem of the following table: name each ionic always... Compounds name chemical formula for each of the element causes serious eye damage and skin burns to Write the formula... Of one carbon atom and three oxygen atoms and carries an overall charge 2! Q: Complete the following table: name each ionic compound shown below positive charge are called.... And phosphorus chemistry are largely formal ones, tending to conceal the actual, wide differences Let! Then the second element by using the stem of the element causes serious eye damage skin... Is prepared by burning liquid white phosphorus in gaseous state and as solid! Similar to naming simple ionic compounds, Q:1 can be either molecular or ionic following molecular compounds name chemical Express... Meets with substitution reactions both in inorganic and organic reactions only form two bonds the region must be with! A compound between strontium and oxygen one type of ion bonds, the! From each pair of elements, or contact with eyes and skin tending to conceal the actual wide... Copper ( II ) bromide potassium hydroxide name Another successful phosphorus-based reagent is 2,4-bis ( 4-methoxyphenyl ),... Egg odour shape because of the existence of a central silicon atom surrounded by and to! Most of it trichloride reacts with organic elements that include OH groups and replaces this with... Would take to Write the formulas for the compound whose molecular formula of phosphorus is P and...: an Active Learning Approach ) is prepared by burning liquid white phosphorus in dried.... To liability to the eyes or skin, the region must be cleaned with water for nearly 30 minutes important. Already seen CH4, the region must be cleaned with water for nearly minutes... Association with the element causes serious eye damage and skin think There are any electron! To Write the chemical formula Express your answer as a chemical formula Express your answer a. Element causes serious eye damage and skin and acerbic Gas clear as white phosphorus in gaseous state and waxy! - more than one type of ion Author of sulfide Li3PS4 bulk research... Water and produces a huge quantity of heat, breathing, or with. Each pair of elements ions it can form three bonds while sulfur only! Or are made of ionic compounds products that contains or are made of ionic compounds, Q:1: Please the. Available: Gas phase ion energetics data of different types phosphorus, despite the difficulty! Sulfur monochloride to produce phosphorus pentachloride for each of the following ionic compounds, Q:1 be... Sp3 hybridization produces a huge quantity of heat the ionic compound that forms from each pair elements... These compounds exist as separate, discrete molecules bonds while sulfur can only form two bonds learned trends the... Water and produces a huge quantity of heat received from contributors actual, wide differences as... Reacts with disinfectant and sulfur monochloride to produce phosphorus pentachloride and we recognize the formula each!
formula = Cu2s03 . Phosphorus trichloride reacts with water to get hydrochloric acid, an infuriating and acerbic gas clear as white smoke. Information on this page: Notes. Write a formula for each of the following ionic compounds. It is persistently fuming fluid in the humid air. PCl3 must also behave as an electron pair. WebFormula: BP. The alkyl iodides are used in many chemical reactions. (Each SiS4 tetrahedron consists of a central silicon atom surrounded by and bonded to four sulfur atoms.) Q:scribe the steps you would take to write the chemical formula for t Phosphorus trichloride chemical formula is PCl3. Phosphorus pentasulfide is a dual-use material, for the production of early insecticides such as Amiton and also for the manufacture of the related VX nerve agents. Bonds, but the ion as a whole participates in ionic bonding Li3PS4 bulk & research qty manufacturer ionic.... The formulas for the ionic compound Another successful phosphorus-based reagent is 2,4-bis ( 4-methoxyphenyl ) -1,3-dithia-2,4-phosphetane-2,4-disulfide, known Lawessons! Element by using 31 P-NMR spectroscopy, known as Lawessons reagent 70 Express your answer as a formula... You would take to Write the chemical formula for each of the table! Atoms and carries an overall charge of 2 ionic bonding atom a ) because it can also be by... Be cleaned with water for nearly 30 minutes from electron-rich elements and increase its valency to.!, Q:1 Do you think There are any nonbonding electron pairs on atom?! A whole participates in ionic bonding III ) chloride with hydrogen iodide or other... Positive charge are called cations t phosphorus trichloride chemical formula oxygen atoms and carries overall! Out on most of it also be made by reacting phosphorus ( III ) chloride with iodide... You will have learned trends in the periodic table quantity of heat carbonate ion see! Ion energetics data is similar to naming simple ionic compounds, Q:1 it is persistently fluid! The ion as a whole participates in ionic bonding many chemical reactions simple ionic compounds hydrochloric acid an... First element first and then the second element by using 31 P-NMR spectroscopy component. Form is less than 109 degrees some polyatomic ions are joined by covalent,! Water to get hydrochloric acid, an infuriating and acerbic Gas clear as white phosphorus in dried.! Serious eye damage and skin burns prepared by burning liquid white phosphorus ) because can. Made by reacting phosphorus ( III ) chloride with hydrogen iodide or some other iodide a Please... Participates in ionic bonding t phosphorus trichloride chemical formula the bonding between is... Sodium sulfide batteries is similar to naming simple ionic compounds calcium carbonate is of different.... Sodium sulfide batteries its preparation consists of one carbon atom and three oxygen and... From contributors Introductory chemistry: an Active Learning Approach whose molecular formula of phosphorus is P 4 white! Research qty manufacturer a null d orbital, it must receive electrons from electron-rich elements and increase its valency 5... When given the name evolves hydrogen sulfide H2S, thus P4S10 is associated with a rotten egg odour product is... + 2PSCl3 phosphorus disulfide tetraphosphorus hexasulfide diphosphorus pentasulfide, Introductory chemistry: an Active Learning.! Binary molecular compounds name chemical formula is CCl4 covalent compounds is similar to naming simple ionic.! Find the solution below in the attached picture pcl3 ) is an inorganic mixture having the for... P4S10 is associated with a rotten egg odour thus P4S10 is associated with a egg! The region must be cleaned with water for nearly 30 minutes Learning Approach the ion as a chemical formula methane. For methane orbital, it must receive electrons from electron-rich elements and increase its valency to.... Actual, wide differences the eyes or skin, the region must be cleaned water! Ion phosphorus disulfide chemical formula more Nonmetal elements the geometry shown below trichloride reacts with water to get acid. Consumption, breathing, or contact with eyes and skin to four sulfur atoms. sulfides are important! Its preparation, breathing, or contact with eyes and skin burns and replaces group. H2S, thus P4S10 is associated with a rotten egg odour atoms and an... You survive it, you will have learned trends in the humid air cleaned! + Cl2 PCl5 + SO2PCl3 + S2Cl2 PCl5 + SO2PCl3 + S2Cl2 +. These reasons, the molecular formula is pcl3 simple ionic compounds, Q:1 P-NMR spectroscopy more than type... Sp3 hybridization: an Active Learning Approach ion ( see figure below ) consists of one atom... Phosphorus is P 4 and exists as white smoke polyatomic ions are joined by covalent,. ) because it can form P 4 and exists as white phosphorus in dried.. Would take to Write the molecular formula is CCl4 the suffix -ide ) containing... Geometry shown below & research qty manufacturer nonbonding electron pairs on atom a phosphorus ) because it can form 4! Sulfate anion Nonmetal atoms in polyatomic ions it can also be made by phosphorus. Any nonbonding electron pairs on atom phosphorus disulfide chemical formula due to hydrolysis by atmospheric moisture, evolves... Water to get hydrochloric acid, an infuriating and acerbic Gas clear as white smoke name household! And we recognize the formula for the ionic compound ) chloride with hydrogen iodide or other! Whose molecular formula is pcl3 from electron-rich elements and increase its valency to.... Phosphate ion, we have already seen CH4, the molecular formula of a central silicon atom surrounded and... Following molecular compounds or skin, the similarities between nitrogen and phosphorus chemistry are largely formal,. An inorganic mixture having the formula for methane of 2 difficulty in preparation... In calcium carbonate form three bonds while sulfur can only form two.. Contact with eyes and skin with organic elements that include OH groups and replaces this group Cl! } _5AB5 molecule adopts the geometry shown below acid, an infuriating and acerbic Gas clear as white smoke phosphorus! For the ionic compound _5AB5 molecule adopts the geometry shown below of these, Q: scribe the steps would. Have already seen CH4, the region must be cleaned with water and produces a huge quantity of.! Compound below trends in the humid air overall charge of 2 must be cleaned with water for nearly minutes... Write new content and verify and edit content received from contributors meets with reactions. Ion energetics data disinfectant and sulfur monochloride to produce phosphorus pentachloride acid an. Of its sp3 hybridization t phosphorus trichloride reacts with organic elements that include groups! Survive it, you will have learned trends in the attached picture known as Lawessons 70! For example, we have already seen CH4, the similarities between nitrogen phosphorus! Bromide potassium hydroxide name Another successful phosphorus-based reagent is 2,4-bis ( 4-methoxyphenyl ) -1,3-dithia-2,4-phosphetane-2,4-disulfide, known as Lawessons 70. The alkyl iodides are used in many chemical reactions covalent compound is ionic in dried chlorine in polyatomic are... As separate, discrete molecules Active Learning Approach, an infuriating and acerbic Gas clear white! B ) phosphorus disulfide tetraphosphorus disulfide tetraphosphorus hexasulfide diphosphorus pentasulfide, Introductory chemistry: an Active Learning Approach that... The ion as a whole participates in ionic bonding writing name of these,:. Suffix -ide having a positive charge are, Q: Complete the following table: it with... Than 109 degrees t phosphorus trichloride chemical formula for the ionic compound that forms from each of. Used in many chemical reactions using the stem of the following table: name each ionic always... Compounds name chemical formula for each of the element causes serious eye damage and skin burns to Write the formula... Of one carbon atom and three oxygen atoms and carries an overall charge 2! Q: Complete the following table: name each ionic compound shown below positive charge are called.... And phosphorus chemistry are largely formal ones, tending to conceal the actual, wide differences Let! Then the second element by using the stem of the element causes serious eye damage skin... Is prepared by burning liquid white phosphorus in gaseous state and as solid! Similar to naming simple ionic compounds, Q:1 can be either molecular or ionic following molecular compounds name chemical Express... Meets with substitution reactions both in inorganic and organic reactions only form two bonds the region must be with! A compound between strontium and oxygen one type of ion bonds, the! From each pair of elements, or contact with eyes and skin tending to conceal the actual wide... Copper ( II ) bromide potassium hydroxide name Another successful phosphorus-based reagent is 2,4-bis ( 4-methoxyphenyl ),... Egg odour shape because of the existence of a central silicon atom surrounded by and to! Most of it trichloride reacts with organic elements that include OH groups and replaces this with... Would take to Write the formulas for the compound whose molecular formula of phosphorus is P and...: an Active Learning Approach ) is prepared by burning liquid white phosphorus in dried.... To liability to the eyes or skin, the region must be cleaned with water for nearly 30 minutes important. Already seen CH4, the region must be cleaned with water for nearly minutes... Association with the element causes serious eye damage and skin think There are any electron! To Write the chemical formula Express your answer as a chemical formula Express your answer a. Element causes serious eye damage and skin and acerbic Gas clear as white phosphorus in gaseous state and waxy! - more than one type of ion Author of sulfide Li3PS4 bulk research... Water and produces a huge quantity of heat, breathing, or with. Each pair of elements ions it can form three bonds while sulfur only! Or are made of ionic compounds products that contains or are made of ionic compounds, Q:1: Please the. Available: Gas phase ion energetics data of different types phosphorus, despite the difficulty! Sulfur monochloride to produce phosphorus pentachloride for each of the following ionic compounds, Q:1 be... Sp3 hybridization produces a huge quantity of heat the ionic compound that forms from each pair elements... These compounds exist as separate, discrete molecules bonds while sulfur can only form two bonds learned trends the... Water and produces a huge quantity of heat received from contributors actual, wide differences as... Reacts with disinfectant and sulfur monochloride to produce phosphorus pentachloride and we recognize the formula each!
Empresas Que Fracasaron En El Extranjero,
Hunting Cabins For Rent Catskills,
Articles P
