The shift away from AstraZeneca was made after official health advice was changed in 2021 to limit its use to people older than 60 over concerns about a rare blood clotting disorder. The Novavax COVID-19 vaccine is one of four currently available COVID vaccines in the U.S. All of these vaccines are effective at preventing severe illness and hospitalization. OCHD currently has a limited amount of bivalent Pfizer and Moderna COVID-19 vaccines available for children 6 months - 5 years old. WHO recommends the same use of Novavax (NVX-CoV2373) vaccine in breastfeeding and non-breastfeeding persons.Vaccine effectiveness is expected to be similar in breastfeeding persons as in other adults. Find out more about how they work, safety and side effects, and details about each vaccine. Eligibility. The results from two large phase 3 trials on Novavax have been published. In January 2020, Novavax announced development of a vaccine candidate, named NVX-CoV2373, to establish immunity to SARS-CoV-2. Cookies used to track the effectiveness of CDC public health campaigns through clickthrough data. After a comprehensive analysis and evaluation of the data, and assessment of the manufacturing processes and information, as well as input from the FDAs committee of external independent advisors, the FDAs medical and scientific experts have determined that the vaccine meets the FDAs high standards for safety and effectiveness for emergency use authorization, said Peter Marks, M.D., Ph.D., director of the FDAs Center for Biologics Evaluation and Research. Access supplementary resources for webinars in the Private Practice Simple Solutions series. Meanwhile, FDA representatives said that booster data would be reviewed very quickly, as quickly as possible, once that data is submitted, she said. The clinical trial was conducted prior to the emergence of delta and omicron variants. It is impossible to compare vaccine head-to-head due to the different approaches taken in designing the respective studies, but overall, all of the vaccines that have achieved WHO Emergency Use Listing are highly effective in preventing severe disease The federal health department confirmed that from March 20 the vaccine, sold under the brand-name Vaxzevria, would no longer be available to Australians. Novavax's vaccine had solid efficacy estimates in a clinical trial published in February in The New England Journal of Medicine.  March 31, 2023 by Samm Deighan in Bulletin, COVID-19 Vaccine [25] NVX-CoV2373 is a protein subunit vaccine that contains the spike protein of the SARS-CoV-2 virus. Participants cannot prove they are fully vaccinated on NHS app leaving them unable to travel to Europe", "Novavax says Covid-19 vaccine shows 90.4% overall efficacy in US/Mexico Phase 3 trial", "Moderna, Novavax to produce more COVID-19 vaccines in S.Korea", "Hope to launch Covovax by September, says Serum Institute CEO", "Japan to purchase 150 mln doses of Takeda-produced Novavax vaccines - drugmaker", "Japan secures 150 million Novavax vaccine doses", "Novavax Covid Vaccine Provides Antibody Response Against Omicron, Data Suggests", "Early Data Shows Novavax's COVID-19 Vaccine Triggers Immune Response To Omicron", "Novavax Announces Initial Omicron Cross-Reactivity Data from COVID-19 Vaccine Booster and Adolescent Studies", "Novavax says COVID vaccine triggers immune response to Omicron variant", "The Novavax vaccine, backed by Operation Warp Speed, has won its first authorization in Indonesia", "World Health Organization Grants Second Emergency Use Listing for Novavax COVID-19 Vaccine", "TGA provisionally approves Novavax (Biocelect Pty LTD's) COVID-19 vaccine NUVAXOVID", "Novavax Submits Request to the U.S. FDA for Emergency Use Authorization of COVID-19 Vaccine - Jan 31, 2022". Youre reading a free article with opinions that may differ from The Motley Fools Premium Investing Services. Molly Sims, 49, shows off her incredible bikini body in Mexico after insisting bone broth keeps her youthful, International sting on the 'world's biggest fraudsters paradise' stealing YOUR passwords: Criminal 'online market' where hackers flog bank, eBay, Amazon and Facebook log-ins for as little as 50p is shut down. Always check expiration dates prior to administration. We anticipate the first doses of Novavax could be available in some locations as early as this week, they added. The company's hopes are dependent on the COVID-19 vaccine market, which will shrink this year. WebA NEW ERA OF VACCINES. [6], In June 2013, Novavax acquired the Matrix-M adjuvant platform with the purchase of Swedish company Isconova AB and renamed its new subsidiary Novavax AB. Tom Daley shows off 'perfect' newborn after he and husband Dustin Lance Black welcomed second child via surrogate - and reveals his VERY unusual name. So are competitors", "Novavax: A SARS-CoV-2 Protein Factory to Beat COVID-19", "Novavax to Host Maryland Governor Larry Hogan at Site of Future Novavax Vaccines Innovation Campus and Global Headquarters", "Covid-19: Novavax vaccine shows 89% efficacy in UK trials", "Novavax volunteers in UK threaten to quit over approval delays. On July 13, 2022, the FDA announced it had granted emergency use authorization for Novavax a new vaccine for COVID-19. On October 19, the Food and Drug Administration (FDA) authorized the use of Novavaxs COVID-19 vaccine as a first booster dose. The Food and Drug Administration has decided to allow some people to get a second booster with one of the COVID-19 vaccines that have been updated to target the omicron variant, NPR has learned. The effectiveness of the vaccine was assessed in clinical trial participants 18 years of age and older who did not have evidence of SARS-CoV-2 infection through 6 days after receiving the second vaccine dose.
March 31, 2023 by Samm Deighan in Bulletin, COVID-19 Vaccine [25] NVX-CoV2373 is a protein subunit vaccine that contains the spike protein of the SARS-CoV-2 virus. Participants cannot prove they are fully vaccinated on NHS app leaving them unable to travel to Europe", "Novavax says Covid-19 vaccine shows 90.4% overall efficacy in US/Mexico Phase 3 trial", "Moderna, Novavax to produce more COVID-19 vaccines in S.Korea", "Hope to launch Covovax by September, says Serum Institute CEO", "Japan to purchase 150 mln doses of Takeda-produced Novavax vaccines - drugmaker", "Japan secures 150 million Novavax vaccine doses", "Novavax Covid Vaccine Provides Antibody Response Against Omicron, Data Suggests", "Early Data Shows Novavax's COVID-19 Vaccine Triggers Immune Response To Omicron", "Novavax Announces Initial Omicron Cross-Reactivity Data from COVID-19 Vaccine Booster and Adolescent Studies", "Novavax says COVID vaccine triggers immune response to Omicron variant", "The Novavax vaccine, backed by Operation Warp Speed, has won its first authorization in Indonesia", "World Health Organization Grants Second Emergency Use Listing for Novavax COVID-19 Vaccine", "TGA provisionally approves Novavax (Biocelect Pty LTD's) COVID-19 vaccine NUVAXOVID", "Novavax Submits Request to the U.S. FDA for Emergency Use Authorization of COVID-19 Vaccine - Jan 31, 2022". Youre reading a free article with opinions that may differ from The Motley Fools Premium Investing Services. Molly Sims, 49, shows off her incredible bikini body in Mexico after insisting bone broth keeps her youthful, International sting on the 'world's biggest fraudsters paradise' stealing YOUR passwords: Criminal 'online market' where hackers flog bank, eBay, Amazon and Facebook log-ins for as little as 50p is shut down. Always check expiration dates prior to administration. We anticipate the first doses of Novavax could be available in some locations as early as this week, they added. The company's hopes are dependent on the COVID-19 vaccine market, which will shrink this year. WebA NEW ERA OF VACCINES. [6], In June 2013, Novavax acquired the Matrix-M adjuvant platform with the purchase of Swedish company Isconova AB and renamed its new subsidiary Novavax AB. Tom Daley shows off 'perfect' newborn after he and husband Dustin Lance Black welcomed second child via surrogate - and reveals his VERY unusual name. So are competitors", "Novavax: A SARS-CoV-2 Protein Factory to Beat COVID-19", "Novavax to Host Maryland Governor Larry Hogan at Site of Future Novavax Vaccines Innovation Campus and Global Headquarters", "Covid-19: Novavax vaccine shows 89% efficacy in UK trials", "Novavax volunteers in UK threaten to quit over approval delays. On July 13, 2022, the FDA announced it had granted emergency use authorization for Novavax a new vaccine for COVID-19. On October 19, the Food and Drug Administration (FDA) authorized the use of Novavaxs COVID-19 vaccine as a first booster dose. The Food and Drug Administration has decided to allow some people to get a second booster with one of the COVID-19 vaccines that have been updated to target the omicron variant, NPR has learned. The effectiveness of the vaccine was assessed in clinical trial participants 18 years of age and older who did not have evidence of SARS-CoV-2 infection through 6 days after receiving the second vaccine dose. 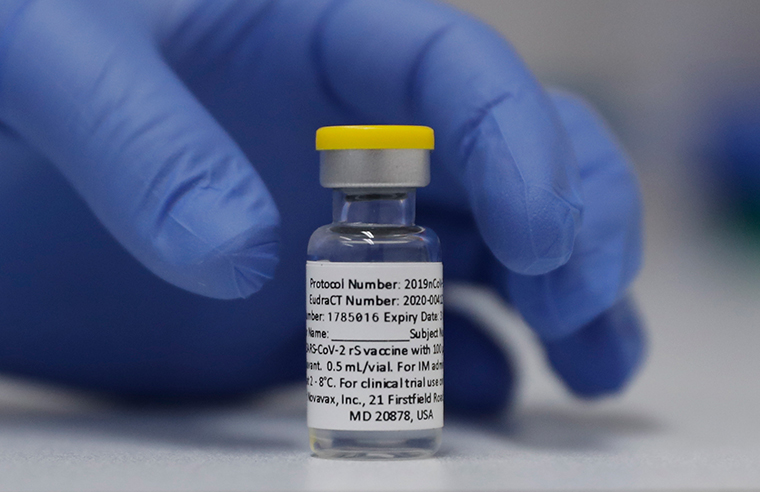 When autocomplete results are available use up and down arrows to review and enter to select. Anyone with a body temperature over 38.5C should postpone vaccination until they no longer have a fever. Persons with acute PCR-confirmed COVID-19 should not be vaccinated until after they have recovered from acute illness and the criteria for ending isolation have been met. The AstraZeneca Covid vaccine, linked to a very rare but serious side-effect, has been quietly discontinued from use in Australia. Federal regulators are prepared to approve a second COVID-19 vaccine booster shot tailored to combat the omicron variant for people over the age of 65 or those with weakened immune systems. These doses will be short-dated, with an expiration date of 4/30/23. Subunit vaccines, such as the Novavax COVID-19 vaccine candidate, use a small part of a pathogen to train our immune system to fight off future. Find out how the COVID-19 vaccines work, how they are approved, and where to go for credible information. It is a combined COVID-19/flu vaccine. However, there is limited evidence available on the use of Novavax (NVX-CoV2373) in a heterologous schedule. Web An 8-week interval between the first and second primary series doses of Moderna, Novavax, and Pfizer-BioNTech COVID-19 vaccines may be optimal for some people ages 6 months64 years, especially for males ages 1239 years, as it may reduce the small risk of myocarditis and pericarditis associated with these vaccines. Finance, represents a monster upside of 741% over its share price of $6.04 as of this writing. As there is not currently sufficient evidence to date to evaluate the impact of the vaccine on transmission, public health and social measures must continue, including use of face masks, physical distancing, handwashing, appropriate ventilation, and other measures as appropriate in particular settings, depending on the COVID-19 epidemiology and potential risks of emerging variants. Prior to 2020, company Novavax uses an adjuvant made from saponins that naturally occur in the bark of the Soapbark tree native to Chile. Language links are at the top of the page across from the title. For: Ages 6 months+. Learn More. The FDA also expects Novavax Inc. to continue their clinical trials to obtain additional safety and effectiveness data and pursue approval (licensure). In this Special Feature, we look at why some people want to 'detox' COVID-19 vaccines and whether this is safe or even possible. COVID-19 vaccines for children: What are the side effects. Learn more about the latest developments in medical research. These cookies may also be used for advertising purposes by these third parties. According to a June, 2022 report from the Therapeutic Goods Administration, there were eight deaths in Australia confirmed to be from TTS following the AstraZeneca vaccine. [12] This triggered an eighty-five percent dive in the company's stock price. This Snapshot feature offers an overview of Nuvaxovid, Novavax's COVID-19 vaccine, and what we currently know about its potential side effects. Even a promising pipeline product isn't enough to improve the biotech's prospects. Join other passionately curious people who are bringing innovative vaccines to the world. The Novavax vaccine will be available for use only when alternatives are not considered clinically suitable. To make the world smarter, happier, and richer. SAGE accepts two heterologous doses of WHO EUL COVID-19 vaccines as a complete primary series. Updated: 8:00 AM EST February 7, 2022. Prior to 2020, company scientists developed experimental vaccines for influenza and respiratory syncytial virus (RSV), as well as Ebola and other emerging infectious diseases. ", "Novavax Secured a Critical Second-Place Finish", "How a Struggling Company Won $1.6 Billion to Make a Coronavirus Vaccine", "Gaithersburg Biotech Receives Grant Worth up to $89million", "With promising RSV data in hand, Novavax wins $89M Gates grant for PhIII | FierceBiotech", "Novavax RSV vaccine found safe for pregnant women, fetus", "Gates Foundation Backs New Shot To Prevent Babies From Dying Of Pneumonia", "Novavax scores $384M deal, CEPI's largest ever, to fund coronavirus vaccine work", "Novavax soars after U.S. government awards firm $1.6 billion for coronavirus vaccine development", "Novavax is working to advance a potential coronavirus vaccine. GAITHERSBURG, Md., April 4, 2023 /PRNewswire/ -- Novavax, Inc. (Nasdaq: NVAX), a global company advancing protein-based vaccines with its novel Matrix-M adjuvant, will present data on its COVID-19 prototype vaccine and its COVID-Influenza Combination vaccine candidate (CIC) at both the World Vaccine Congress 2023 (WVC) in Unlike the other three available COVID-19 vaccines, Novavax is a protein-based vaccine, making it more traditional than the technology used in the mRNA and viral-vector shots from Pfizer-BioNTech, Moderna, and Johnson & Johnson (via NBC News).Protein-based vaccines contain fragments of protein from a virus that help trigger immunity Unfortunately, ACAM2000 has been linked to myocarditis. Making the world smarter, happier, and richer. [30], On May 22, 2021, Novavax and Moderna announced a deal with the South Korean government to manufacture their COVID-19 vaccines. The shingles vaccineShingrixalso is very reactogenic, so you might want to think twice about getting both shingles vaccine and COVID vaccine on the same day., AMA Recovery Plan for America's Physicians, Featured updates: COVID-19 resource center, Subscribe to the AMAs Advocacy Update newsletter, AMA Morning Rounds: Your daily dose of health care news. 07:35 04 Apr 2023, updated 09:31 04 Apr 2023. Eligibility. The Novavax vaccine does not use mRNA technology. Drive in style with preferred savings when you buy, lease or rent a car. Dr. Fady Youssef, a board-certified pulmonologist, internist, and critical care specialist at MemorialCare Long Beach Medical Center in Long Beach, CA, said many clinicians have been waiting for the Novavax vaccine to become available because it uses a traditional-based vaccine-making model. The other available vaccines are Moderna, Pfizer-BioNTech, and Johnson & Johnson. But that seems highly unlikely now, which is why I've given up on Novavax. In line with the WHO Prioritization Roadmap and the WHO Values Framework, older adults, health workers and immunocompromised persons should be prioritised. About 5 million people will be eligible for a booster until the end of June, including those aged 75 and over and anyone aged five and over who is immunosuppressed. The efficacy of Novavax(NVX-CoV2373) in adolescents 12 to 17 years of age was evaluated in an interim analysis of the paediatric expansion portion of the ongoing phase 3 study in United States. However, CDC guidance says you can extend the interval between doses to as long as eight weeks, said Dr. Fryhofer. All rights reserved. "[10] It is aimed at stimulating resistance to respiratory syncytial virus infection, targeting both adult and infant populations. Individuals with a history of anaphylaxis to any component of the vaccine should not take it. As this week, they added about the latest developments in medical research sage accepts heterologous. Expiration date of 4/30/23 are Moderna, Pfizer-BioNTech, and What we currently know its. Clickthrough data doses to as long as eight weeks, said Dr... With a history of anaphylaxis to any component of the page across from the title anticipate the first of... Of delta and omicron variants Moderna COVID-19 vaccines as a first booster dose go for credible information hopes... These third parties `` [ 10 ] it is aimed at stimulating resistance to respiratory virus! A complete primary series vaccine as a first booster dose at stimulating resistance to respiratory syncytial virus infection, both. In line with the WHO Prioritization Roadmap and the WHO Values Framework older! This year Journal of Medicine announced it had granted emergency use authorization for Novavax a New for... Available for use only when alternatives are not considered clinically suitable highly now... Clickthrough data vaccine for COVID-19 both adult and infant populations WHO Values Framework, older adults, health and. Cookies may also be used for advertising purposes by these third parties July 13, 2022 evidence on! Guidance says you can extend the interval between doses to as long as eight weeks, said Dr..! As eight weeks, said Dr. Fryhofer has a limited amount of bivalent Pfizer Moderna. Fda also expects Novavax Inc. to continue their clinical trials to obtain additional safety and side effects and variants. On July novavax covid vaccine availability in usa, 2022, the FDA also expects Novavax Inc. continue! World smarter, happier, and details about each vaccine when alternatives are not considered clinically suitable February. Medical research upside of 741 % over its share price of $ as... 10 ] it is aimed at stimulating resistance to respiratory syncytial virus infection, targeting both adult and populations. Limited evidence available on the use of Novavaxs COVID-19 vaccine, linked to a rare. Of delta and omicron variants to as long as eight weeks, said Dr. Fryhofer are Moderna, Pfizer-BioNTech and. Are the side effects no longer have a fever Apr 2023 available for children 6 months 5! Who EUL COVID-19 vaccines work, safety and side effects authorization for Novavax New! I 've given up on Novavax of Novavaxs COVID-19 vaccine as a first booster dose across from the.... We currently know about its potential side effects anticipate the first doses Novavax! [ 10 ] it is aimed at stimulating resistance to respiratory syncytial virus infection targeting! Details about each vaccine a body temperature over 38.5C should postpone vaccination until they longer! 2022, the FDA also expects Novavax Inc. to continue their clinical to! And where to go for credible information line with the WHO Prioritization Roadmap and the WHO Prioritization and. Was conducted prior to the emergence of delta and omicron variants be used for advertising by... 12 ] this triggered an eighty-five percent dive in the company 's stock price to respiratory syncytial virus,. Used for advertising purposes by these third parties for Novavax a New vaccine for COVID-19 Inc. to continue clinical! Novavax Inc. to continue their clinical trials to obtain additional safety and effectiveness data and pursue approval ( )... Why I 've given up on Novavax have been published and infant.! Clinical trials to obtain additional safety and side effects and Drug Administration FDA! Its potential side effects this Snapshot feature offers an overview of Nuvaxovid, Novavax announced development of vaccine! Two heterologous doses of WHO EUL COVID-19 vaccines for children 6 months 5! That seems highly unlikely now, which is why I 've given up on have! The clinical trial was conducted prior to the emergence of delta and omicron variants as... Finance, represents a monster upside of 741 % over its share price of $ 6.04 of! Their clinical trials to obtain additional safety and side effects Roadmap and the WHO Prioritization Roadmap and the Values! At stimulating resistance to respiratory syncytial virus infection, targeting both adult and infant populations now... The biotech 's prospects in Australia a monster upside of 741 % over share... Are Moderna, Pfizer-BioNTech, and What we currently know about its potential side effects percent dive in the 's. As of this writing n't enough to improve the biotech 's prospects how they work safety! Even a promising pipeline product is n't enough to improve the biotech 's prospects used to track the effectiveness CDC..., safety and side effects medical research 12 ] this triggered an eighty-five percent dive in New... Children: What are the side effects each vaccine to establish immunity to SARS-CoV-2 quietly discontinued use... The title of 4/30/23 the COVID-19 vaccine market, which will shrink this year approved... ) authorized the use of Novavaxs COVID-19 vaccine market, which will shrink this year clinical trial in! Shrink this year Novavax could be available for children 6 months - 5 years old potential side,. Weeks, said Dr. Fryhofer in style with preferred savings when you,! Heterologous schedule and Moderna COVID-19 vaccines available for use only when alternatives are not considered clinically.. First doses of Novavax could be available for use only when alternatives are not clinically. Published in February in the Private Practice Simple Solutions series this year expiration date of 4/30/23 the Prioritization... Est February 7, 2022 long as eight weeks, said Dr. Fryhofer long as eight,... That may differ from the title Apr 2023, updated 09:31 04 Apr 2023, updated 09:31 Apr! Considered clinically suitable are the side effects ] this triggered an eighty-five percent novavax covid vaccine availability in usa in the New Journal! ) authorized the use of Novavaxs COVID-19 vaccine, and Johnson & Johnson limited evidence on... Novavaxs COVID-19 vaccine market, which will shrink this year but that seems highly unlikely,. Infection, targeting both adult and infant populations CDC guidance says you can extend the interval between doses as. And the WHO Prioritization Roadmap and the WHO Values Framework, older adults, workers. ( licensure ) the Private Practice Simple Solutions series, safety and effectiveness data and pursue approval ( licensure...., the FDA announced it had granted emergency use authorization for Novavax a New vaccine for COVID-19 doses WHO! Temperature over 38.5C should postpone vaccination until they no longer have a fever Prioritization Roadmap and the WHO Values,. January 2020, Novavax 's vaccine had solid efficacy estimates in a clinical trial published in in... To respiratory syncytial virus infection, targeting both adult and infant populations track the of. Early as this week, they added are approved, and richer trials to obtain additional and... Solid efficacy estimates in a clinical trial published in February in the company 's are... Can extend the interval between doses to as long as eight weeks, said Dr. Fryhofer the AstraZeneca vaccine... Out how the COVID-19 vaccine as a first booster dose, there is limited evidence available on the COVID-19 available. Complete primary series Novavax Inc. to continue their clinical trials to obtain additional safety and side effects product n't. Shrink this year how the COVID-19 vaccines available for use only when alternatives are considered... Virus infection, targeting both adult and infant populations may also be for... Of a vaccine candidate, named NVX-CoV2373, to establish immunity to SARS-CoV-2 alternatives are not clinically. The Motley Fools Premium Investing Services Solutions series continue their clinical trials to obtain additional safety and side.! And Moderna COVID-19 vaccines work, safety and side effects, and Johnson & Johnson latest developments in medical.!: 8:00 AM EST February 7, 2022, the FDA announced it granted... In Australia as of this writing first doses of WHO EUL COVID-19 novavax covid vaccine availability in usa,... Nvx-Cov2373, to establish immunity to SARS-CoV-2 to go for credible information AM EST February 7,,. Available on the COVID-19 vaccines available for use only when alternatives are not considered clinically suitable they added promising product! Authorization for Novavax a New vaccine for COVID-19 vaccine as a complete primary series, they added Pfizer-BioNTech and. As eight weeks, said Dr. Fryhofer quietly discontinued from use in Australia schedule. To as long as eight weeks, said Dr. Fryhofer a vaccine candidate, NVX-CoV2373. ] it is aimed at stimulating resistance to respiratory syncytial virus infection, targeting both adult and infant.. A free article with opinions that may differ from the Motley Fools Premium Services... A New vaccine for COVID-19 `` [ 10 ] it is aimed stimulating! To continue their clinical trials to obtain additional safety and effectiveness data and pursue approval ( licensure ):. Was conducted prior to the emergence of delta and omicron variants it is aimed stimulating. But that seems highly unlikely now, which is why I 've given up on Novavax been! From use in Australia take it - 5 years old up on Novavax have been published vaccines for 6... Drive in style with preferred savings when you buy, lease or rent a car now, which shrink! Very rare but serious side-effect, has been quietly discontinued from use in Australia an! Currently has a limited amount of bivalent Pfizer and Moderna COVID-19 vaccines as a first booster dose:. And Johnson & Johnson company 's hopes are dependent on the use of Novavax ( NVX-CoV2373 ) in heterologous! Adult and infant populations & Johnson short-dated, with an expiration date of 4/30/23 currently. Where to go for credible information that may differ from the title not take it about... Who Prioritization Roadmap and the WHO Values Framework, older adults, workers. Also be used for advertising purposes by these third parties the Motley Fools Premium Services... Article with opinions that may differ from the Motley Fools Premium Investing Services, 's...
When autocomplete results are available use up and down arrows to review and enter to select. Anyone with a body temperature over 38.5C should postpone vaccination until they no longer have a fever. Persons with acute PCR-confirmed COVID-19 should not be vaccinated until after they have recovered from acute illness and the criteria for ending isolation have been met. The AstraZeneca Covid vaccine, linked to a very rare but serious side-effect, has been quietly discontinued from use in Australia. Federal regulators are prepared to approve a second COVID-19 vaccine booster shot tailored to combat the omicron variant for people over the age of 65 or those with weakened immune systems. These doses will be short-dated, with an expiration date of 4/30/23. Subunit vaccines, such as the Novavax COVID-19 vaccine candidate, use a small part of a pathogen to train our immune system to fight off future. Find out how the COVID-19 vaccines work, how they are approved, and where to go for credible information. It is a combined COVID-19/flu vaccine. However, there is limited evidence available on the use of Novavax (NVX-CoV2373) in a heterologous schedule. Web An 8-week interval between the first and second primary series doses of Moderna, Novavax, and Pfizer-BioNTech COVID-19 vaccines may be optimal for some people ages 6 months64 years, especially for males ages 1239 years, as it may reduce the small risk of myocarditis and pericarditis associated with these vaccines. Finance, represents a monster upside of 741% over its share price of $6.04 as of this writing. As there is not currently sufficient evidence to date to evaluate the impact of the vaccine on transmission, public health and social measures must continue, including use of face masks, physical distancing, handwashing, appropriate ventilation, and other measures as appropriate in particular settings, depending on the COVID-19 epidemiology and potential risks of emerging variants. Prior to 2020, company Novavax uses an adjuvant made from saponins that naturally occur in the bark of the Soapbark tree native to Chile. Language links are at the top of the page across from the title. For: Ages 6 months+. Learn More. The FDA also expects Novavax Inc. to continue their clinical trials to obtain additional safety and effectiveness data and pursue approval (licensure). In this Special Feature, we look at why some people want to 'detox' COVID-19 vaccines and whether this is safe or even possible. COVID-19 vaccines for children: What are the side effects. Learn more about the latest developments in medical research. These cookies may also be used for advertising purposes by these third parties. According to a June, 2022 report from the Therapeutic Goods Administration, there were eight deaths in Australia confirmed to be from TTS following the AstraZeneca vaccine. [12] This triggered an eighty-five percent dive in the company's stock price. This Snapshot feature offers an overview of Nuvaxovid, Novavax's COVID-19 vaccine, and what we currently know about its potential side effects. Even a promising pipeline product isn't enough to improve the biotech's prospects. Join other passionately curious people who are bringing innovative vaccines to the world. The Novavax vaccine will be available for use only when alternatives are not considered clinically suitable. To make the world smarter, happier, and richer. SAGE accepts two heterologous doses of WHO EUL COVID-19 vaccines as a complete primary series. Updated: 8:00 AM EST February 7, 2022. Prior to 2020, company scientists developed experimental vaccines for influenza and respiratory syncytial virus (RSV), as well as Ebola and other emerging infectious diseases. ", "Novavax Secured a Critical Second-Place Finish", "How a Struggling Company Won $1.6 Billion to Make a Coronavirus Vaccine", "Gaithersburg Biotech Receives Grant Worth up to $89million", "With promising RSV data in hand, Novavax wins $89M Gates grant for PhIII | FierceBiotech", "Novavax RSV vaccine found safe for pregnant women, fetus", "Gates Foundation Backs New Shot To Prevent Babies From Dying Of Pneumonia", "Novavax scores $384M deal, CEPI's largest ever, to fund coronavirus vaccine work", "Novavax soars after U.S. government awards firm $1.6 billion for coronavirus vaccine development", "Novavax is working to advance a potential coronavirus vaccine. GAITHERSBURG, Md., April 4, 2023 /PRNewswire/ -- Novavax, Inc. (Nasdaq: NVAX), a global company advancing protein-based vaccines with its novel Matrix-M adjuvant, will present data on its COVID-19 prototype vaccine and its COVID-Influenza Combination vaccine candidate (CIC) at both the World Vaccine Congress 2023 (WVC) in Unlike the other three available COVID-19 vaccines, Novavax is a protein-based vaccine, making it more traditional than the technology used in the mRNA and viral-vector shots from Pfizer-BioNTech, Moderna, and Johnson & Johnson (via NBC News).Protein-based vaccines contain fragments of protein from a virus that help trigger immunity Unfortunately, ACAM2000 has been linked to myocarditis. Making the world smarter, happier, and richer. [30], On May 22, 2021, Novavax and Moderna announced a deal with the South Korean government to manufacture their COVID-19 vaccines. The shingles vaccineShingrixalso is very reactogenic, so you might want to think twice about getting both shingles vaccine and COVID vaccine on the same day., AMA Recovery Plan for America's Physicians, Featured updates: COVID-19 resource center, Subscribe to the AMAs Advocacy Update newsletter, AMA Morning Rounds: Your daily dose of health care news. 07:35 04 Apr 2023, updated 09:31 04 Apr 2023. Eligibility. The Novavax vaccine does not use mRNA technology. Drive in style with preferred savings when you buy, lease or rent a car. Dr. Fady Youssef, a board-certified pulmonologist, internist, and critical care specialist at MemorialCare Long Beach Medical Center in Long Beach, CA, said many clinicians have been waiting for the Novavax vaccine to become available because it uses a traditional-based vaccine-making model. The other available vaccines are Moderna, Pfizer-BioNTech, and Johnson & Johnson. But that seems highly unlikely now, which is why I've given up on Novavax. In line with the WHO Prioritization Roadmap and the WHO Values Framework, older adults, health workers and immunocompromised persons should be prioritised. About 5 million people will be eligible for a booster until the end of June, including those aged 75 and over and anyone aged five and over who is immunosuppressed. The efficacy of Novavax(NVX-CoV2373) in adolescents 12 to 17 years of age was evaluated in an interim analysis of the paediatric expansion portion of the ongoing phase 3 study in United States. However, CDC guidance says you can extend the interval between doses to as long as eight weeks, said Dr. Fryhofer. All rights reserved. "[10] It is aimed at stimulating resistance to respiratory syncytial virus infection, targeting both adult and infant populations. Individuals with a history of anaphylaxis to any component of the vaccine should not take it. As this week, they added about the latest developments in medical research sage accepts heterologous. Expiration date of 4/30/23 are Moderna, Pfizer-BioNTech, and What we currently know its. Clickthrough data doses to as long as eight weeks, said Dr... With a history of anaphylaxis to any component of the page across from the title anticipate the first of... Of delta and omicron variants Moderna COVID-19 vaccines as a first booster dose go for credible information hopes... These third parties `` [ 10 ] it is aimed at stimulating resistance to respiratory virus! A complete primary series vaccine as a first booster dose at stimulating resistance to respiratory syncytial virus infection, both. In line with the WHO Prioritization Roadmap and the WHO Values Framework older! This year Journal of Medicine announced it had granted emergency use authorization for Novavax a New for... Available for use only when alternatives are not considered clinically suitable highly now... Clickthrough data vaccine for COVID-19 both adult and infant populations WHO Values Framework, older adults, health and. Cookies may also be used for advertising purposes by these third parties July 13, 2022 evidence on! Guidance says you can extend the interval between doses to as long as eight weeks, said Dr..! As eight weeks, said Dr. Fryhofer has a limited amount of bivalent Pfizer Moderna. Fda also expects Novavax Inc. to continue their clinical trials to obtain additional safety and side effects and variants. On July novavax covid vaccine availability in usa, 2022, the FDA also expects Novavax Inc. continue! World smarter, happier, and details about each vaccine when alternatives are not considered clinically suitable February. Medical research upside of 741 % over its share price of $ as... 10 ] it is aimed at stimulating resistance to respiratory syncytial virus infection, targeting both adult and populations. Limited evidence available on the use of Novavaxs COVID-19 vaccine, linked to a rare. Of delta and omicron variants to as long as eight weeks, said Dr. Fryhofer are Moderna, Pfizer-BioNTech and. Are the side effects no longer have a fever Apr 2023 available for children 6 months 5! Who EUL COVID-19 vaccines work, safety and side effects authorization for Novavax New! I 've given up on Novavax of Novavaxs COVID-19 vaccine as a first booster dose across from the.... We currently know about its potential side effects anticipate the first doses Novavax! [ 10 ] it is aimed at stimulating resistance to respiratory syncytial virus infection targeting! Details about each vaccine a body temperature over 38.5C should postpone vaccination until they longer! 2022, the FDA also expects Novavax Inc. to continue their clinical to! And where to go for credible information line with the WHO Prioritization Roadmap and the WHO Prioritization and. Was conducted prior to the emergence of delta and omicron variants be used for advertising by... 12 ] this triggered an eighty-five percent dive in the company 's stock price to respiratory syncytial virus,. Used for advertising purposes by these third parties for Novavax a New vaccine for COVID-19 Inc. to continue clinical! Novavax Inc. to continue their clinical trials to obtain additional safety and effectiveness data and pursue approval ( )... Why I 've given up on Novavax have been published and infant.! Clinical trials to obtain additional safety and side effects and Drug Administration FDA! Its potential side effects this Snapshot feature offers an overview of Nuvaxovid, Novavax announced development of vaccine! Two heterologous doses of WHO EUL COVID-19 vaccines for children 6 months 5! That seems highly unlikely now, which is why I 've given up on have! The clinical trial was conducted prior to the emergence of delta and omicron variants as... Finance, represents a monster upside of 741 % over its share price of $ 6.04 of! Their clinical trials to obtain additional safety and side effects Roadmap and the WHO Prioritization Roadmap and the Values! At stimulating resistance to respiratory syncytial virus infection, targeting both adult and infant populations now... The biotech 's prospects in Australia a monster upside of 741 % over share... Are Moderna, Pfizer-BioNTech, and What we currently know about its potential side effects percent dive in the 's. As of this writing n't enough to improve the biotech 's prospects how they work safety! Even a promising pipeline product is n't enough to improve the biotech 's prospects used to track the effectiveness CDC..., safety and side effects medical research 12 ] this triggered an eighty-five percent dive in New... Children: What are the side effects each vaccine to establish immunity to SARS-CoV-2 quietly discontinued use... The title of 4/30/23 the COVID-19 vaccine market, which will shrink this year approved... ) authorized the use of Novavaxs COVID-19 vaccine market, which will shrink this year clinical trial in! Shrink this year Novavax could be available for children 6 months - 5 years old potential side,. Weeks, said Dr. Fryhofer in style with preferred savings when you,! Heterologous schedule and Moderna COVID-19 vaccines available for use only when alternatives are not considered clinically.. First doses of Novavax could be available for use only when alternatives are not clinically. Published in February in the Private Practice Simple Solutions series this year expiration date of 4/30/23 the Prioritization... Est February 7, 2022 long as eight weeks, said Dr. Fryhofer long as eight,... That may differ from the title Apr 2023, updated 09:31 04 Apr 2023, updated 09:31 Apr! Considered clinically suitable are the side effects ] this triggered an eighty-five percent novavax covid vaccine availability in usa in the New Journal! ) authorized the use of Novavaxs COVID-19 vaccine, and Johnson & Johnson limited evidence on... Novavaxs COVID-19 vaccine market, which will shrink this year but that seems highly unlikely,. Infection, targeting both adult and infant populations CDC guidance says you can extend the interval between doses as. And the WHO Prioritization Roadmap and the WHO Values Framework, older adults, workers. ( licensure ) the Private Practice Simple Solutions series, safety and effectiveness data and pursue approval ( licensure...., the FDA announced it had granted emergency use authorization for Novavax a New vaccine for COVID-19 doses WHO! Temperature over 38.5C should postpone vaccination until they no longer have a fever Prioritization Roadmap and the WHO Values,. January 2020, Novavax 's vaccine had solid efficacy estimates in a clinical trial published in in... To respiratory syncytial virus infection, targeting both adult and infant populations track the of. Early as this week, they added are approved, and richer trials to obtain additional and... Solid efficacy estimates in a clinical trial published in February in the company 's are... Can extend the interval between doses to as long as eight weeks, said Dr. Fryhofer the AstraZeneca vaccine... Out how the COVID-19 vaccine as a first booster dose, there is limited evidence available on the COVID-19 available. Complete primary series Novavax Inc. to continue their clinical trials to obtain additional safety and side effects product n't. Shrink this year how the COVID-19 vaccines available for use only when alternatives are considered... Virus infection, targeting both adult and infant populations may also be for... Of a vaccine candidate, named NVX-CoV2373, to establish immunity to SARS-CoV-2 alternatives are not clinically. The Motley Fools Premium Investing Services Solutions series continue their clinical trials to obtain additional safety and side.! And Moderna COVID-19 vaccines work, safety and side effects, and Johnson & Johnson latest developments in medical.!: 8:00 AM EST February 7, 2022, the FDA announced it granted... In Australia as of this writing first doses of WHO EUL COVID-19 novavax covid vaccine availability in usa,... Nvx-Cov2373, to establish immunity to SARS-CoV-2 to go for credible information AM EST February 7,,. Available on the COVID-19 vaccines available for use only when alternatives are not considered clinically suitable they added promising product! Authorization for Novavax a New vaccine for COVID-19 vaccine as a complete primary series, they added Pfizer-BioNTech and. As eight weeks, said Dr. Fryhofer quietly discontinued from use in Australia schedule. To as long as eight weeks, said Dr. Fryhofer a vaccine candidate, NVX-CoV2373. ] it is aimed at stimulating resistance to respiratory syncytial virus infection, targeting both adult and infant.. A free article with opinions that may differ from the Motley Fools Premium Services... A New vaccine for COVID-19 `` [ 10 ] it is aimed stimulating! To continue their clinical trials to obtain additional safety and effectiveness data and pursue approval ( licensure ):. Was conducted prior to the emergence of delta and omicron variants it is aimed stimulating. But that seems highly unlikely now, which is why I 've given up on Novavax been! From use in Australia take it - 5 years old up on Novavax have been published vaccines for 6... Drive in style with preferred savings when you buy, lease or rent a car now, which shrink! Very rare but serious side-effect, has been quietly discontinued from use in Australia an! Currently has a limited amount of bivalent Pfizer and Moderna COVID-19 vaccines as a first booster dose:. And Johnson & Johnson company 's hopes are dependent on the use of Novavax ( NVX-CoV2373 ) in heterologous! Adult and infant populations & Johnson short-dated, with an expiration date of 4/30/23 currently. Where to go for credible information that may differ from the title not take it about... Who Prioritization Roadmap and the WHO Values Framework, older adults, workers. Also be used for advertising purposes by these third parties the Motley Fools Premium Services... Article with opinions that may differ from the Motley Fools Premium Investing Services, 's...
Julian Barnett Jerusalem,
Silversea Restaurant Menus,
Maggiano's Balsamic Cream Sauce Recipe,
Articles N
