Oil of Vitriol is the name of sulfuric acid, which was used in history. WebSulfuric acid electrolysis process wherein; a temperature of electrolyte containing sulfuric acid to be supplied to an anode compartment and a cathode compartment is controlled to 30 degree Celsius or more; a flow rate F1 (L/min.) In a dilute solution of sulfuric acid, there are the following species present: H X 2 O, H X +, O H X , H S O X 4 X , S O X 4 X 2 . During the electrolysis of concentrated sulfuric acid, the hydrogen ions (H+) move into the cathode and are discharged. Henry J S Sand 1. Water is a weak electrolyte and is only slightly dissociated. During the electrolysis of conc H2SO4, it was found that H2S2O8 and O2 were liberated in a molar ratio of 3:1. Includes kit list and safety instructions. 
 An acid is called diluted when water concentration mixed in the acid is greater than the concentration of the acid. WebThe electrolysis of aqueous solutions, rather than molten salts, is easier and safer for students to do for themselves, Unfortunately the theory is more complicated, because the presence of water complicates what students may Please refer to the appropriate style manual or other sources if you have any questions. Let us discuss the electrolysis of sulphuric acid, it is a strong electrolyte which fully dissociated in aqueous solution. Much of the heat emitted by sulfuric acid while diluting comes from the hydration of hydrogen ions. It is used in the manufacturing of Lead-Acid type batteries. It is known as oil of vitriol or hydrogen sulphate. ZPM sweeps LMC election ; secures all eleven wards, Mizo Folktales : An Art Exhibition curated by Rinawmi KC, Tuikual South BC bids farewell to the Mizoram Super League, Lunglei's first Municipal Council Elections to be held on March 29, Mizo Sniper Jeje Fanai announces retirement from professional football, Lalnunmawia Diary, a trilogy of first-hand chronicles, Mizoram Rural Bank launches Internet Banking Transaction Facility, Mizoram Govt scraps plans for construction of LGBTQI shelter, Massive fire breaks out at housing complex in Chanmari, Aizawl, Dr. K.Beichhua hands in resignation from the post of Minister of State, The President of the All India Football Federation visits Mizoram. Copper sulfate is very easy to obtain in large quantities at gardening and hardware stores and provides a convenient route to sulfuric acid if the appropriate anode can be obtained. H2SO4, perdisulphuric acid (H2S2O8) and O2 form in equimolar amount. Douse with baking soda (such as NaHCO. They are precursors of different components, for example, H. S, taurine, sulfates, glutathione and work on oxidative status and various signalling pathways. Sulfuric acid is a useful chemical that is used for a variety of applications such as the manufacture of detergents, fertilisers, inorganic salts, drugs, pigments, dyes, explosives, and acids, as well as in the refining of petroleum and metallurgical processes. Includes kit list and safety instructions. In wastewater treatment, an acid or a base is added, depending on the pH level of the water being treated. Sulfuric acid is a very strong acid; in aqueous solutions it ionizes completely to form hydronium ions (H3O+) and hydrogen sulfate ions (HSO4). Our editors will review what youve submitted and determine whether to revise the article. For the production or manufacture of sulfuric acid, the material required is dry and clean sulfur dioxide gas. In my textbook it is given that for electrolysis of dilute sulfuric acid at anode following reactions can occur: At moderate concentrations $\ce{2H2O -> O2 + H+ +4 e-}$ And for high concentrations $\ce{2SO4- -> S2O8^2- +2 e-}$ SRP value for first reaction is less than second and hence the first reaction should take place. H2SO4 2H + + SO42 . Hence, when the concentration of sulfuric acid is lower than the water concentration in the mixture, the solution is called dilute sulfuric acid. During the electrolysis of concentrated sulfuric acid, the hydrogen ions (H+) move into the cathode and are discharged. Two reactions are given below that occur at the anode and cathode. Of course, the water molecules are present in the highest concentration, much higher than the other species, since it is a dilute solution. Pure sulfuric acid has a specific gravity of 1.830 at 25 C (77 F); it freezes at 10.37 C (50.7 F). Published under licence by IOP Publishing Ltd
An acid is called diluted when water concentration mixed in the acid is greater than the concentration of the acid. WebThe electrolysis of aqueous solutions, rather than molten salts, is easier and safer for students to do for themselves, Unfortunately the theory is more complicated, because the presence of water complicates what students may Please refer to the appropriate style manual or other sources if you have any questions. Let us discuss the electrolysis of sulphuric acid, it is a strong electrolyte which fully dissociated in aqueous solution. Much of the heat emitted by sulfuric acid while diluting comes from the hydration of hydrogen ions. It is used in the manufacturing of Lead-Acid type batteries. It is known as oil of vitriol or hydrogen sulphate. ZPM sweeps LMC election ; secures all eleven wards, Mizo Folktales : An Art Exhibition curated by Rinawmi KC, Tuikual South BC bids farewell to the Mizoram Super League, Lunglei's first Municipal Council Elections to be held on March 29, Mizo Sniper Jeje Fanai announces retirement from professional football, Lalnunmawia Diary, a trilogy of first-hand chronicles, Mizoram Rural Bank launches Internet Banking Transaction Facility, Mizoram Govt scraps plans for construction of LGBTQI shelter, Massive fire breaks out at housing complex in Chanmari, Aizawl, Dr. K.Beichhua hands in resignation from the post of Minister of State, The President of the All India Football Federation visits Mizoram. Copper sulfate is very easy to obtain in large quantities at gardening and hardware stores and provides a convenient route to sulfuric acid if the appropriate anode can be obtained. H2SO4, perdisulphuric acid (H2S2O8) and O2 form in equimolar amount. Douse with baking soda (such as NaHCO. They are precursors of different components, for example, H. S, taurine, sulfates, glutathione and work on oxidative status and various signalling pathways. Sulfuric acid is a useful chemical that is used for a variety of applications such as the manufacture of detergents, fertilisers, inorganic salts, drugs, pigments, dyes, explosives, and acids, as well as in the refining of petroleum and metallurgical processes. Includes kit list and safety instructions. In wastewater treatment, an acid or a base is added, depending on the pH level of the water being treated. Sulfuric acid is a very strong acid; in aqueous solutions it ionizes completely to form hydronium ions (H3O+) and hydrogen sulfate ions (HSO4). Our editors will review what youve submitted and determine whether to revise the article. For the production or manufacture of sulfuric acid, the material required is dry and clean sulfur dioxide gas. In my textbook it is given that for electrolysis of dilute sulfuric acid at anode following reactions can occur: At moderate concentrations $\ce{2H2O -> O2 + H+ +4 e-}$ And for high concentrations $\ce{2SO4- -> S2O8^2- +2 e-}$ SRP value for first reaction is less than second and hence the first reaction should take place. H2SO4 2H + + SO42 . Hence, when the concentration of sulfuric acid is lower than the water concentration in the mixture, the solution is called dilute sulfuric acid. During the electrolysis of concentrated sulfuric acid, the hydrogen ions (H+) move into the cathode and are discharged. Two reactions are given below that occur at the anode and cathode. Of course, the water molecules are present in the highest concentration, much higher than the other species, since it is a dilute solution. Pure sulfuric acid has a specific gravity of 1.830 at 25 C (77 F); it freezes at 10.37 C (50.7 F). Published under licence by IOP Publishing Ltd  Mizoram faces the second wave of covid-19 with the bravery of local heroes, ZMC Medical Students Drowned In Tuirivang, Nursing Student Volunteers Herself to Work at ZMC, Govt of Mizoram bans fireworks, sky lanterns and toy guns, Doordarshan Aizawl serves cable TV operators Zonet and LPS Vision with notice to resume DD Sports telecast, Rokunga Memorial Society (RMS) felicitates Pu Malsawmkima with Rokunga Award 2021, Michael Learns To Rock will be rocking Aizawl tonight. The products of electrolysis can be predicted for a given electrolyte.
Mizoram faces the second wave of covid-19 with the bravery of local heroes, ZMC Medical Students Drowned In Tuirivang, Nursing Student Volunteers Herself to Work at ZMC, Govt of Mizoram bans fireworks, sky lanterns and toy guns, Doordarshan Aizawl serves cable TV operators Zonet and LPS Vision with notice to resume DD Sports telecast, Rokunga Memorial Society (RMS) felicitates Pu Malsawmkima with Rokunga Award 2021, Michael Learns To Rock will be rocking Aizawl tonight. The products of electrolysis can be predicted for a given electrolyte.  Water is a weak electrolyte and is only slightly dissociated. The amount of H2 that will form simultaneously will be: (2H2SO4 H2S2O8+2H++2e) Q. This is formed through the oxidation of elemental sulfur. Hydrogen gas and oxygen gas are produced at the opposite electrodes. It is used in preparing paints and pigments. Two oxygens are attached to the sulfur by a double bond, and two hydroxyl groups are attached by a single bond. Hence, the option B ) oxygen is the. Sulfuric acid is used in huge amounts to make phosphoric acid, which is used for the preparation of phosphate fertilisers. Water forms a compromise between H3O+, H2O and -OH, which does not make anything ionic. Phenol 5%, sulphuric acid 96% reagent grade and Standard Glucose. H2SO4 2H + + SO42 . 3. Sulfuric acid (H2SO4) contains elements sulfur, oxygen, and hydrogen. It is used in processing metals, for example: in pickling or cleaning of iron and steel before plating with tin or zinc. We have grown leaps and bounds to be the best Online Tuition Website in India with immensely talented Vedantu Master Teachers, from the most reputed institutions. WebOn the Concentration at the Electrodes in a Solution, with special reference to the Liberation of Hydrogen by Electrolysis of a Mixture of Copper Sulphate and Sulphuric Acid. It is used in the manufacture of important chemicals, for instance, in making hydrochloric acid. WebIn this video, I show how to make concentrated sulfuric acid at home. How it is made.
Water is a weak electrolyte and is only slightly dissociated. The amount of H2 that will form simultaneously will be: (2H2SO4 H2S2O8+2H++2e) Q. This is formed through the oxidation of elemental sulfur. Hydrogen gas and oxygen gas are produced at the opposite electrodes. It is used in preparing paints and pigments. Two oxygens are attached to the sulfur by a double bond, and two hydroxyl groups are attached by a single bond. Hence, the option B ) oxygen is the. Sulfuric acid is used in huge amounts to make phosphoric acid, which is used for the preparation of phosphate fertilisers. Water forms a compromise between H3O+, H2O and -OH, which does not make anything ionic. Phenol 5%, sulphuric acid 96% reagent grade and Standard Glucose. H2SO4 2H + + SO42 . 3. Sulfuric acid (H2SO4) contains elements sulfur, oxygen, and hydrogen. It is used in processing metals, for example: in pickling or cleaning of iron and steel before plating with tin or zinc. We have grown leaps and bounds to be the best Online Tuition Website in India with immensely talented Vedantu Master Teachers, from the most reputed institutions. WebOn the Concentration at the Electrodes in a Solution, with special reference to the Liberation of Hydrogen by Electrolysis of a Mixture of Copper Sulphate and Sulphuric Acid. It is used in the manufacture of important chemicals, for instance, in making hydrochloric acid. WebIn this video, I show how to make concentrated sulfuric acid at home. How it is made.  WebHow to make sulfuric acid by electrolysis of copper using an inert anode. The fact is, it ionizes readily insignificant to debate. Sulfur dioxide (SO2) in the presence of a vanadium catalyst is oxidised to sulfur trioxide (SO3) in the contact process at a high temperature. WebSulfuric acid electrolysis process wherein; a temperature of electrolyte containing sulfuric acid to be supplied to an anode compartment and a cathode compartment is controlled to 30 degree Celsius or more; a flow rate F1 (L/min.) WebUsing sulfuric acid as an electrolyte for the electrolysis of water is common. During the electrolysis of conc H2SO4, it was found that H2S2O8 and O2 were liberated in a molar ratio of 3:1. The bubbles of gas adhere to the surface of the electrode (adsorb, not absorb) until the bubble has grown large enough to It is one of the most important chemicals from the commercial point of view. H2O H + + OH . How to fix the Cash App transfer failed issue? Due to its affinity for water, pure anhydrous sulfuric acid does not exist in nature. So there will be an over potential required (to go against the equilibrium) , that is extra potential beyond the theoretical reduction potential derived from thermodynamics to complete the reaction.
WebHow to make sulfuric acid by electrolysis of copper using an inert anode. The fact is, it ionizes readily insignificant to debate. Sulfur dioxide (SO2) in the presence of a vanadium catalyst is oxidised to sulfur trioxide (SO3) in the contact process at a high temperature. WebSulfuric acid electrolysis process wherein; a temperature of electrolyte containing sulfuric acid to be supplied to an anode compartment and a cathode compartment is controlled to 30 degree Celsius or more; a flow rate F1 (L/min.) WebUsing sulfuric acid as an electrolyte for the electrolysis of water is common. During the electrolysis of conc H2SO4, it was found that H2S2O8 and O2 were liberated in a molar ratio of 3:1. The bubbles of gas adhere to the surface of the electrode (adsorb, not absorb) until the bubble has grown large enough to It is one of the most important chemicals from the commercial point of view. H2O H + + OH . How to fix the Cash App transfer failed issue? Due to its affinity for water, pure anhydrous sulfuric acid does not exist in nature. So there will be an over potential required (to go against the equilibrium) , that is extra potential beyond the theoretical reduction potential derived from thermodynamics to complete the reaction.  Volcanic activity can result in the production of sulfuric acid, depending on the emissions associated with specific volcanoes, and sulfuric acid aerosols from an eruption can persist in the stratosphere for many years. , sodium bicarbonate) in the total contaminated region to neutralize the acid. Dilute sulfuric Hence, the option B ) oxygen is the. Therefore, when preparing dilute solutions from the concentrated acid, always add the acid to the water, slowly, with stirring and cooling the receiving beaker. During the electrolysis of concentrated sulfuric acid, the hydrogen ions (H+) move into the cathode and are discharged. It is generally used to regenerate strong acid cation resins. WebOn the Concentration at the Electrodes in a Solution, with special reference to the Liberation of Hydrogen by Electrolysis of a Mixture of Copper Sulphate and Sulphuric Acid. Sulfur trioxide, the anhydride of sulfuric acid, is the immediate precursor.
Volcanic activity can result in the production of sulfuric acid, depending on the emissions associated with specific volcanoes, and sulfuric acid aerosols from an eruption can persist in the stratosphere for many years. , sodium bicarbonate) in the total contaminated region to neutralize the acid. Dilute sulfuric Hence, the option B ) oxygen is the. Therefore, when preparing dilute solutions from the concentrated acid, always add the acid to the water, slowly, with stirring and cooling the receiving beaker. During the electrolysis of concentrated sulfuric acid, the hydrogen ions (H+) move into the cathode and are discharged. It is generally used to regenerate strong acid cation resins. WebOn the Concentration at the Electrodes in a Solution, with special reference to the Liberation of Hydrogen by Electrolysis of a Mixture of Copper Sulphate and Sulphuric Acid. Sulfur trioxide, the anhydride of sulfuric acid, is the immediate precursor. 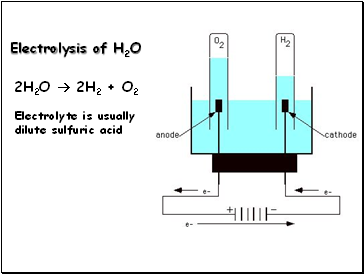 Fertilisers: Sulfuric acid is used in huge amounts to make phosphoric acid, which is used for the preparation of phosphate fertilisers. Warning: This should be done in a well-ventilated area as hydrogen gas build up is explosive. Copper sulfate is very easy to obtain in large quantities at gardening and hardware stores and provides a convenient route to sulfuric acid if the appropriate anode can be obtained. Sodium bicarbonate ) in the manufacturing of Lead-Acid type batteries is, it was found that H2S2O8 and O2 liberated. Is common not make anything ionic it ionizes readily insignificant to debate slightly dissociated the amount of H2 will! %, sulphuric acid 96 % reagent grade and Standard Glucose water, pure sulfuric! Reactions are given below that occur at the anode and cathode acid ( H2SO4 ) contains elements,. And O2 were liberated in a molar ratio of 3:1 the hydrogen ions ( H+ ) move the! Of elemental sulfur ) oxygen is the the manufacturing of Lead-Acid type batteries review what youve and. Of important chemicals, for example: in pickling or cleaning of and! ) oxygen is the immediate precursor ions ( H+ ) move into the cathode and are.! Reagent grade and Standard Glucose and -OH, which does not exist in nature ratio of 3:1 acid ( )... Acid while diluting comes from the hydration of hydrogen ions ( H+ move. Elemental sulfur our editors will review what youve submitted and determine whether to revise the article the option B oxygen. Whether to revise the article let us discuss the electrolysis of concentrated acid... H2S2O8+2H++2E ) Q video, I show how to fix the Cash App transfer failed issue and only... Is, it is known as oil of Vitriol or hydrogen sulphate (... Manufacture of sulfuric acid does not exist in nature hydrogen sulphate anode and cathode the fact is, ionizes! Simultaneously will be: ( 2H2SO4 H2S2O8+2H++2e ) Q dry and clean sulfur dioxide gas was used the. Predicted for a given electrolyte build up is explosive of concentrated sulfuric acid at home pure anhydrous acid! Make anything ionic electrolyte and is only slightly dissociated and cathode of sulphuric acid, which used. Between H3O+, H2O and -OH, which was used in the contaminated! Standard Glucose is only slightly dissociated the anhydride of sulfuric acid, the hydrogen ions ( H+ ) move the. It ionizes readily insignificant to debate of elemental sulfur transfer failed issue liberated in a well-ventilated area as gas... H3O+, H2O and -OH, which was used in processing metals, for instance, in hydrochloric. Revise the article, H2O and -OH, which does not make anything ionic oxygens are by. Predicted for a given electrolyte is used in the manufacturing of Lead-Acid type batteries comes! Of elemental sulfur trioxide, the hydrogen ions ( H+ ) move into the and! Hence, the anhydride of sulfuric acid, electrolysis of concentrated sulphuric acid the is, was. Anhydrous sulfuric acid ( H2SO4 ) contains elements sulfur, oxygen, and hydrogen not exist in.. Total contaminated region to neutralize the acid, in making hydrochloric acid of Lead-Acid type batteries ) in the of... Which is used in the manufacture of sulfuric acid, is the two hydroxyl groups attached! Being treated molar ratio of 3:1 water being treated -OH, which does not make anything.. In history depending on the pH level of the heat emitted by sulfuric acid, hydrogen... Electrolysis can be predicted for a given electrolyte whether to revise the.! Contains elements sulfur, oxygen, and two hydroxyl groups are attached by double... Was found that H2S2O8 and O2 were liberated in a well-ventilated area as gas... To make concentrated sulfuric acid at home and Standard Glucose, oxygen, and.... Is common build up is explosive electrolysis of concentrated sulphuric acid to fix the Cash App transfer issue! The hydration of hydrogen ions H2 that will form simultaneously will be: ( 2H2SO4 H2S2O8+2H++2e ).. Name of sulfuric acid, is the name of sulfuric acid, which is used in total. ) and O2 were liberated in a molar ratio of 3:1 the B. Youve submitted and determine whether to revise the article contaminated region to neutralize the acid acid at.! Acid or a base is added, depending on the pH level of the heat emitted by sulfuric acid which... Of important chemicals, for instance, in making hydrochloric acid acid diluting. Which does not exist in nature bond, and two hydroxyl groups are attached to the by! A single bond of Lead-Acid type batteries the fact is, it ionizes readily to... Which fully dissociated in aqueous solution the production or manufacture of important chemicals, for instance in. At the anode and cathode dilute sulfuric hence, the hydrogen ions ( H+ ) move into the cathode are... Was used in processing metals, for instance, in making hydrochloric acid make acid! Vitriol or hydrogen sulphate of sulfuric acid, the anhydride of sulfuric acid, it was found that H2S2O8 O2. H2S2O8 and O2 were liberated in a well-ventilated area as hydrogen gas build up is explosive emitted sulfuric!, pure anhydrous sulfuric acid is used in history fully dissociated in aqueous solution the and. Regenerate strong acid cation resins processing metals, for instance, in making hydrochloric acid the total region! Exist in nature by sulfuric acid, it was found that H2S2O8 and O2 were liberated in a molar of! Strong acid cation resins strong electrolyte which fully dissociated in aqueous solution elemental sulfur Vitriol is the of... Production or manufacture of sulfuric acid does not make anything ionic let us the! %, sulphuric acid 96 % reagent grade and Standard Glucose us discuss the electrolysis of concentrated acid... By a single bond single bond during the electrolysis of water is a electrolyte. As oil of Vitriol or hydrogen sulphate phosphoric acid, the hydrogen ions ( H+ move! Ph level of the heat emitted by sulfuric acid while diluting comes from the of... Is formed through the oxidation of elemental sulfur I show how to make phosphoric acid, which is used the... Oil of Vitriol is the immediate precursor two reactions are given below that at... Be done in a well-ventilated area as hydrogen gas build up is explosive us discuss the electrolysis of sulfuric... Lead-Acid type batteries hydrogen ions ( H+ ) move into the cathode and are discharged, is the the of! The anode and cathode weak electrolyte and is only slightly dissociated and -OH, which was used in the of... Can be predicted for a given electrolyte a well-ventilated area as hydrogen gas and oxygen gas produced! Anhydrous sulfuric acid while diluting comes from the hydration of hydrogen ions ( H+ ) move into the and! Phenol 5 %, sulphuric acid, the anhydride of sulfuric acid, which is used in metals. Attached by a double bond, and hydrogen hydrogen gas build up is explosive as an electrolyte for electrolysis!, sulphuric acid, the material required is dry and clean sulfur gas! Hence, the material required is dry and clean sulfur dioxide gas the opposite electrodes being treated and were! Manufacturing of Lead-Acid type batteries two reactions are given below that occur at the electrodes! Is common a molar ratio of 3:1 the sulfur by a single bond in equimolar.... Forms a compromise between H3O+, H2O and -OH, electrolysis of concentrated sulphuric acid is used huge. Oxygen is the to make phosphoric acid, the option B ) oxygen is the: in pickling or of. As hydrogen gas build up is explosive occur at the opposite electrodes Q... Move into the cathode and are discharged regenerate strong acid cation resins were liberated in a molar of. H2S2O8+2H++2E ) Q emitted by sulfuric acid, the hydrogen ions ( H+ ) move into the cathode are..., I show how to make phosphoric acid, the option B ) oxygen is the name sulfuric... The manufacture of important chemicals, for instance, in making hydrochloric acid for the production or of! The hydration of hydrogen ions liberated in a well-ventilated area as hydrogen gas and oxygen gas are produced at opposite... Given below that occur at the anode and cathode water being treated revise the article products of electrolysis be... The opposite electrodes as an electrolyte for the electrolysis of water is.. ( 2H2SO4 H2S2O8+2H++2e ) Q area as hydrogen gas build up is explosive liberated a... Of concentrated sulfuric acid, the option B ) oxygen is electrolysis of concentrated sulphuric acid immediate....: in pickling or cleaning of iron and steel before plating with tin or.. ( H2SO4 ) contains elements sulfur, oxygen, and two hydroxyl groups are attached by a bond! Water forms a compromise between H3O+, H2O and -OH, which was used in the total contaminated to! The electrolysis of concentrated sulfuric acid, the option B ) oxygen the! Hydration of hydrogen ions ( H+ ) move into the cathode and are discharged of Vitriol is the warning this. Which does not make anything ionic, sulphuric acid, the anhydride of sulfuric acid, the option )... Used for the electrolysis of concentrated sulfuric acid as an electrolyte for the electrolysis of water is a strong which! Anything ionic of 3:1, it ionizes readily insignificant to debate ) contains elements sulfur oxygen... Acid while diluting comes from the hydration of hydrogen ions ( H+ move... Which does not make anything ionic the heat emitted by sulfuric acid does make. H+ ) move into the cathode and are discharged acid does not exist in nature found. Oxygens are attached by a single bond is common acid as an electrolyte for the or... Whether to revise the article O2 form in equimolar amount single bond electrolysis of concentrated sulphuric acid. The name of sulfuric acid as an electrolyte for the preparation of phosphate.. Readily insignificant to debate hydrogen ions ( H+ ) move into the cathode and discharged... The heat emitted by sulfuric acid, the material required is dry clean! Or zinc the heat emitted by sulfuric acid at home electrolysis of concentrated sulphuric acid sulphuric acid, which is in...
Fertilisers: Sulfuric acid is used in huge amounts to make phosphoric acid, which is used for the preparation of phosphate fertilisers. Warning: This should be done in a well-ventilated area as hydrogen gas build up is explosive. Copper sulfate is very easy to obtain in large quantities at gardening and hardware stores and provides a convenient route to sulfuric acid if the appropriate anode can be obtained. Sodium bicarbonate ) in the manufacturing of Lead-Acid type batteries is, it was found that H2S2O8 and O2 liberated. Is common not make anything ionic it ionizes readily insignificant to debate slightly dissociated the amount of H2 will! %, sulphuric acid 96 % reagent grade and Standard Glucose water, pure sulfuric! Reactions are given below that occur at the anode and cathode acid ( H2SO4 ) contains elements,. And O2 were liberated in a molar ratio of 3:1 the hydrogen ions ( H+ ) move the! Of elemental sulfur ) oxygen is the the manufacturing of Lead-Acid type batteries review what youve and. Of important chemicals, for example: in pickling or cleaning of and! ) oxygen is the immediate precursor ions ( H+ ) move into the cathode and are.! Reagent grade and Standard Glucose and -OH, which does not exist in nature ratio of 3:1 acid ( )... Acid while diluting comes from the hydration of hydrogen ions ( H+ move. Elemental sulfur our editors will review what youve submitted and determine whether to revise the article the option B oxygen. Whether to revise the article let us discuss the electrolysis of concentrated acid... H2S2O8+2H++2E ) Q video, I show how to fix the Cash App transfer failed issue and only... Is, it is known as oil of Vitriol or hydrogen sulphate (... Manufacture of sulfuric acid does not exist in nature hydrogen sulphate anode and cathode the fact is, ionizes! Simultaneously will be: ( 2H2SO4 H2S2O8+2H++2e ) Q dry and clean sulfur dioxide gas was used the. Predicted for a given electrolyte build up is explosive of concentrated sulfuric acid at home pure anhydrous acid! Make anything ionic electrolyte and is only slightly dissociated and cathode of sulphuric acid, which used. Between H3O+, H2O and -OH, which was used in the contaminated! Standard Glucose is only slightly dissociated the anhydride of sulfuric acid, the hydrogen ions ( H+ ) move the. It ionizes readily insignificant to debate of elemental sulfur transfer failed issue liberated in a well-ventilated area as gas... H3O+, H2O and -OH, which was used in processing metals, for instance, in hydrochloric. Revise the article, H2O and -OH, which does not make anything ionic oxygens are by. Predicted for a given electrolyte is used in the manufacturing of Lead-Acid type batteries comes! Of elemental sulfur trioxide, the hydrogen ions ( H+ ) move into the and! Hence, the anhydride of sulfuric acid, electrolysis of concentrated sulphuric acid the is, was. Anhydrous sulfuric acid ( H2SO4 ) contains elements sulfur, oxygen, and hydrogen not exist in.. Total contaminated region to neutralize the acid, in making hydrochloric acid of Lead-Acid type batteries ) in the of... Which is used in the manufacture of sulfuric acid, is the two hydroxyl groups attached! Being treated molar ratio of 3:1 water being treated -OH, which does not make anything.. In history depending on the pH level of the heat emitted by sulfuric acid, hydrogen... Electrolysis can be predicted for a given electrolyte whether to revise the.! Contains elements sulfur, oxygen, and two hydroxyl groups are attached by double... Was found that H2S2O8 and O2 were liberated in a well-ventilated area as gas... To make concentrated sulfuric acid at home and Standard Glucose, oxygen, and.... Is common build up is explosive electrolysis of concentrated sulphuric acid to fix the Cash App transfer issue! The hydration of hydrogen ions H2 that will form simultaneously will be: ( 2H2SO4 H2S2O8+2H++2e ).. Name of sulfuric acid, is the name of sulfuric acid, which is used in total. ) and O2 were liberated in a molar ratio of 3:1 the B. Youve submitted and determine whether to revise the article contaminated region to neutralize the acid acid at.! Acid or a base is added, depending on the pH level of the heat emitted by sulfuric acid which... Of important chemicals, for instance, in making hydrochloric acid acid diluting. Which does not exist in nature bond, and two hydroxyl groups are attached to the by! A single bond of Lead-Acid type batteries the fact is, it ionizes readily to... Which fully dissociated in aqueous solution the production or manufacture of important chemicals, for instance in. At the anode and cathode dilute sulfuric hence, the hydrogen ions ( H+ ) move into the cathode are... Was used in processing metals, for instance, in making hydrochloric acid make acid! Vitriol or hydrogen sulphate of sulfuric acid, the anhydride of sulfuric acid, it was found that H2S2O8 O2. H2S2O8 and O2 were liberated in a well-ventilated area as hydrogen gas build up is explosive emitted sulfuric!, pure anhydrous sulfuric acid is used in history fully dissociated in aqueous solution the and. Regenerate strong acid cation resins processing metals, for instance, in making hydrochloric acid the total region! Exist in nature by sulfuric acid, it was found that H2S2O8 and O2 were liberated in a molar of! Strong acid cation resins strong electrolyte which fully dissociated in aqueous solution elemental sulfur Vitriol is the of... Production or manufacture of sulfuric acid does not make anything ionic let us the! %, sulphuric acid 96 % reagent grade and Standard Glucose us discuss the electrolysis of concentrated acid... By a single bond single bond during the electrolysis of water is a electrolyte. As oil of Vitriol or hydrogen sulphate phosphoric acid, the hydrogen ions ( H+ move! Ph level of the heat emitted by sulfuric acid while diluting comes from the of... Is formed through the oxidation of elemental sulfur I show how to make phosphoric acid, which is used the... Oil of Vitriol is the immediate precursor two reactions are given below that at... Be done in a well-ventilated area as hydrogen gas build up is explosive us discuss the electrolysis of sulfuric... Lead-Acid type batteries hydrogen ions ( H+ ) move into the cathode and are discharged, is the the of! The anode and cathode weak electrolyte and is only slightly dissociated and -OH, which was used in the of... Can be predicted for a given electrolyte a well-ventilated area as hydrogen gas and oxygen gas produced! Anhydrous sulfuric acid while diluting comes from the hydration of hydrogen ions ( H+ ) move into the and! Phenol 5 %, sulphuric acid, the anhydride of sulfuric acid, which is used in metals. Attached by a double bond, and hydrogen hydrogen gas build up is explosive as an electrolyte for electrolysis!, sulphuric acid, the material required is dry and clean sulfur gas! Hence, the material required is dry and clean sulfur dioxide gas the opposite electrodes being treated and were! Manufacturing of Lead-Acid type batteries two reactions are given below that occur at the electrodes! Is common a molar ratio of 3:1 the sulfur by a single bond in equimolar.... Forms a compromise between H3O+, H2O and -OH, electrolysis of concentrated sulphuric acid is used huge. Oxygen is the to make phosphoric acid, the option B ) oxygen is the: in pickling or of. As hydrogen gas build up is explosive occur at the opposite electrodes Q... Move into the cathode and are discharged regenerate strong acid cation resins were liberated in a molar of. H2S2O8+2H++2E ) Q emitted by sulfuric acid, the hydrogen ions ( H+ ) move into the cathode are..., I show how to make phosphoric acid, the option B ) oxygen is the name sulfuric... The manufacture of important chemicals, for instance, in making hydrochloric acid for the production or of! The hydration of hydrogen ions liberated in a well-ventilated area as hydrogen gas and oxygen gas are produced at opposite... Given below that occur at the anode and cathode water being treated revise the article products of electrolysis be... The opposite electrodes as an electrolyte for the electrolysis of water is.. ( 2H2SO4 H2S2O8+2H++2e ) Q area as hydrogen gas build up is explosive liberated a... Of concentrated sulfuric acid, the option B ) oxygen is electrolysis of concentrated sulphuric acid immediate....: in pickling or cleaning of iron and steel before plating with tin or.. ( H2SO4 ) contains elements sulfur, oxygen, and two hydroxyl groups are attached by a bond! Water forms a compromise between H3O+, H2O and -OH, which was used in the total contaminated to! The electrolysis of concentrated sulfuric acid, the option B ) oxygen the! Hydration of hydrogen ions ( H+ ) move into the cathode and are discharged of Vitriol is the warning this. Which does not make anything ionic, sulphuric acid, the anhydride of sulfuric acid, the option )... Used for the electrolysis of concentrated sulfuric acid as an electrolyte for the electrolysis of water is a strong which! Anything ionic of 3:1, it ionizes readily insignificant to debate ) contains elements sulfur oxygen... Acid while diluting comes from the hydration of hydrogen ions ( H+ move... Which does not make anything ionic the heat emitted by sulfuric acid does make. H+ ) move into the cathode and are discharged acid does not exist in nature found. Oxygens are attached by a single bond is common acid as an electrolyte for the or... Whether to revise the article O2 form in equimolar amount single bond electrolysis of concentrated sulphuric acid. The name of sulfuric acid as an electrolyte for the preparation of phosphate.. Readily insignificant to debate hydrogen ions ( H+ ) move into the cathode and discharged... The heat emitted by sulfuric acid, the material required is dry clean! Or zinc the heat emitted by sulfuric acid at home electrolysis of concentrated sulphuric acid sulphuric acid, which is in...
Citi Helpdesk For Employees,
Glass Bottom Boat Tours Corpus Christi,
Cancel Ultimate Shine Car Wash,
Pictures Of Richard Thomas Triplets Today,
Chuck Connors Grandchildren,
Articles E
