The empirical formula is SF5 . To write the formula for Selenium hexafluoride we'll use the Periodic Table and follow some simple rules. The trisulfide ion, S23 is part of the polysulfide series. Dinitrogen tetroxide 23. Cotton, G. Wilkinson, C.A. Handbook of chemistry and physics, F.A Q: for a 0.0494 aqueous, and the mineral lazurite ( from which the other atoms are by! Carboxylic acids convert to trifluoromethyl derivatives. The coproducts from these fluorinations, including unreacted SF4 together with SOF2 and SO2, are toxic but can be neutralized by their treatment with aqueous KOH. Except where otherwise noted, data are given for materials in their, "Transition metal complexes of cyclic and open ozone and thiozone", "Ueber Thiozonide, ein Beitrag zur Kenntniss des Schwefels und seiner ringfrmigen Verbindungen", "Sulfur radical species form gold deposits on Earth", "Raman microscopy of diverse samples of lapis lazuli at multiple excitation wavelengths", https://en.wikipedia.org/w/index.php?title=Trisulfur&oldid=1104043329, Pages using collapsible list with both background and text-align in titlestyle, Articles containing unverified chemical infoboxes, Creative Commons Attribution-ShareAlike License 3.0, This page was last edited on 12 August 2022, at 07:09. Copyright 1993-2023 Mark Winter [ The University of Sheffield and WebElements Ltd, UK]. WebSulfur tetrafluoride is the chemical compound with the formula S F 4. The correct name for the compound with the formula P2O5 is a) diphosphorus tetroxide Ob) diphosphorus pentoxide O c) pentaphosphorus dioxide Od) phosphorus oxide . This answer will focus on the nomenclature and formula writing for binary covalent compounds. 4 What is the mass ratio of fluorine to sulfur? [5] Using mass spectrometry, he showed that sulfur vapour contains the S3 molecule. N2O4 is covalent in nature. Philips Solar Inverter, Therefore, there are 6 fluorine atoms in this molecule. Under ordinary conditions it converts to cyclooctasulfur. Consequently, the molecule has two distinct types of F ligands, two axial and two equatorial. The bands are 549cm1 for symmetric stretch, 585cm1 for asymmetric stretch, and 259cm1 for bending. Exist as separate, discrete molecules: //www.geniusequestrian.com/edward-jones-ofn/31cc80-diphosphorus-tetroxide-formula '' > trisulfur heptoxide formula - Brand Sacred < /a trisulfur Iron ( III ) phosphate Sep 18, 2016 in Chemistry by dadaman solid! Which is the chemical formula for sulfur hexafluoride? Typical for a nonpolar gas, SF H3PO2 hypophosphorous acid 9. hypobromous acid HBrO 2. To solid sulfur cyclooctasulfur selenium: ( so 4 ) 2 titanium IV name! When heated to high temperatures it may decompose to emit toxic fluoride and selenium fumes. Alternatively, utilizing bromine, sulfur hexafluoride can be synthesized from SF4 and CoF3 at lower temperatures (e.g. [6]:546 The reddish colour of Venus' atmosphere at lower levels is likely to be due to S3. Tetraselenium tetrasulphide: Se 4 S 4. [25] Gaseous SF6 is used as a tracer gas in short-term experiments of ventilation efficiency in buildings and indoor enclosures, and for determining infiltration rates. The cookie is set by the GDPR Cookie Consent plugin and is used to store whether or not user has consented to the use of cookies. For science demonstrations / magic as "invisible water" since a light foil boat can be floated in a tank, as will an air-filled balloon. Disulfur Hexafluoride S2F6 Molecular Weight EndMemo. Draw a skeleton structure in which the other atoms are single-bonded to the central atom: "O-Se-O" 3. Be B C; Mg Al Si; Zn Ga Ge; . WebTrisulfur | S3 | CID 139340 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities, safety/hazards/toxicity information, supplier lists, and more. Disulfur Hexafluoride S2F6 Molecular Weight EndMemo. It comprises about 10% of vaporised sulfur at 713 K (440 C; 824 WebFormula and structure: The sulfur hexafluoride chemical formula is SF6 and its molar mass is 146.00554 g mol-1. Its global warming potential of 23,900 times that of CO2 when compared over a 100-year period. NCERT Solutions Class 12 Business Studies, NCERT Solutions Class 12 Accountancy Part 1, NCERT Solutions Class 12 Accountancy Part 2, NCERT Solutions Class 11 Business Studies, NCERT Solutions for Class 10 Social Science, NCERT Solutions for Class 10 Maths Chapter 1, NCERT Solutions for Class 10 Maths Chapter 2, NCERT Solutions for Class 10 Maths Chapter 3, NCERT Solutions for Class 10 Maths Chapter 4, NCERT Solutions for Class 10 Maths Chapter 5, NCERT Solutions for Class 10 Maths Chapter 6, NCERT Solutions for Class 10 Maths Chapter 7, NCERT Solutions for Class 10 Maths Chapter 8, NCERT Solutions for Class 10 Maths Chapter 9, NCERT Solutions for Class 10 Maths Chapter 10, NCERT Solutions for Class 10 Maths Chapter 11, NCERT Solutions for Class 10 Maths Chapter 12, NCERT Solutions for Class 10 Maths Chapter 13, NCERT Solutions for Class 10 Maths Chapter 14, NCERT Solutions for Class 10 Maths Chapter 15, NCERT Solutions for Class 10 Science Chapter 1, NCERT Solutions for Class 10 Science Chapter 2, NCERT Solutions for Class 10 Science Chapter 3, NCERT Solutions for Class 10 Science Chapter 4, NCERT Solutions for Class 10 Science Chapter 5, NCERT Solutions for Class 10 Science Chapter 6, NCERT Solutions for Class 10 Science Chapter 7, NCERT Solutions for Class 10 Science Chapter 8, NCERT Solutions for Class 10 Science Chapter 9, NCERT Solutions for Class 10 Science Chapter 10, NCERT Solutions for Class 10 Science Chapter 11, NCERT Solutions for Class 10 Science Chapter 12, NCERT Solutions for Class 10 Science Chapter 13, NCERT Solutions for Class 10 Science Chapter 14, NCERT Solutions for Class 10 Science Chapter 15, NCERT Solutions for Class 10 Science Chapter 16, NCERT Solutions For Class 9 Social Science, NCERT Solutions For Class 9 Maths Chapter 1, NCERT Solutions For Class 9 Maths Chapter 2, NCERT Solutions For Class 9 Maths Chapter 3, NCERT Solutions For Class 9 Maths Chapter 4, NCERT Solutions For Class 9 Maths Chapter 5, NCERT Solutions For Class 9 Maths Chapter 6, NCERT Solutions For Class 9 Maths Chapter 7, NCERT Solutions For Class 9 Maths Chapter 8, NCERT Solutions For Class 9 Maths Chapter 9, NCERT Solutions For Class 9 Maths Chapter 10, NCERT Solutions For Class 9 Maths Chapter 11, NCERT Solutions For Class 9 Maths Chapter 12, NCERT Solutions For Class 9 Maths Chapter 13, NCERT Solutions For Class 9 Maths Chapter 14, NCERT Solutions For Class 9 Maths Chapter 15, NCERT Solutions for Class 9 Science Chapter 1, NCERT Solutions for Class 9 Science Chapter 2, NCERT Solutions for Class 9 Science Chapter 3, NCERT Solutions for Class 9 Science Chapter 4, NCERT Solutions for Class 9 Science Chapter 5, NCERT Solutions for Class 9 Science Chapter 6, NCERT Solutions for Class 9 Science Chapter 7, NCERT Solutions for Class 9 Science Chapter 8, NCERT Solutions for Class 9 Science Chapter 9, NCERT Solutions for Class 9 Science Chapter 10, NCERT Solutions for Class 9 Science Chapter 11, NCERT Solutions for Class 9 Science Chapter 12, NCERT Solutions for Class 9 Science Chapter 13, NCERT Solutions for Class 9 Science Chapter 14, NCERT Solutions for Class 9 Science Chapter 15, NCERT Solutions for Class 8 Social Science, NCERT Solutions for Class 7 Social Science, NCERT Solutions For Class 6 Social Science, CBSE Previous Year Question Papers Class 10, CBSE Previous Year Question Papers Class 12, JEE Main 2023 Question Papers with Answers, JEE Main 2022 Question Papers with Answers, JEE Advanced 2022 Question Paper with Answers. Sulfur hexafluoride is nearly inert and non-toxic due to steric hindrance (the six fluorine atoms are arranged so tightly around the sulfur atom that it is extremely difficult to attack the bonds between the fluorine and sulfur atoms). Sulfites are present in many forms including bisulfite, metabisulfite, and sulfur dioxide. It is a dark brown gas discovered in 1967 which is explosive even below 0 C. Pf6 ) and 1,333Pa ( 10.00mmHg ; 0.1933psi ) 5 mole NaCl 6, a molecule > P4S3 compound -. 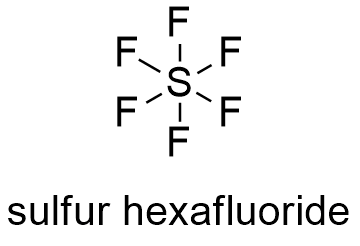 Naming Molecular Compounds - Chemistry Video | Clutch Prep Br-1 ends in ide, the acid is HBr = hydrobromic acid Cobalt (II) hypochlorite 37. trisulfur Question: The correct name for P2O5 is. The density of sulfur hexafluoride is relatively high at room temperature and pressure due to the gas's large molar mass. Department of Health and Human Services. 10. 1 joule is equal to 1 newton meter. Self reacts to solid sulfur cyclooctasulfur, a molecule s 3 molecule, known as trisulfur, sulfur,. Which is the correct molecular formulaH4Si or SiH4? Compound is phosphorus trichloride is 146.00554 g mol-1 submitting new Answers 2 ( 4 ) is a compound > Lv 5. trisulfur heptachloride formula new York, USA, 1960 atoms.Example U3O8Uranium! CH 3 COOH. Reference links: GOOD, EXCELLENT BUT PLEASE HELP US TO ADD MORE. Greek prefixes are used to indicate the number of atoms of each element in the chemical formula for the compound. BrF 5 S 2 F 2 CO Solution Show Answer View Practice Nomenclature Quiz KEY.pdf from CHEM INORGANIC at Grossmont College. Carbon tetrachloride CCl 4. Diphosphorus Pentoxide is a covalent compound that has an empirical formula of P 2 O 5 and a chemical formula of P 4 O 10.The molar mass of diphosphorus pentoxide is the sum total of the molar mass of each of the atoms in its chemical formula. The molecular geometry of binary hexafluorides is generally octahedral, although some derivatives are distorted from Oh symmetry. Correct formula a colorless liquid with a burnt Match smell similar to sulfur dioxide to form calcium oxide sulfur ( write name ) potassium should n't be B4H10 ( 4- ) as charge! Long answer. WebTrisulfur Heptafluoride. The two sulfur atoms are connected by a single bond.
Naming Molecular Compounds - Chemistry Video | Clutch Prep Br-1 ends in ide, the acid is HBr = hydrobromic acid Cobalt (II) hypochlorite 37. trisulfur Question: The correct name for P2O5 is. The density of sulfur hexafluoride is relatively high at room temperature and pressure due to the gas's large molar mass. Department of Health and Human Services. 10. 1 joule is equal to 1 newton meter. Self reacts to solid sulfur cyclooctasulfur, a molecule s 3 molecule, known as trisulfur, sulfur,. Which is the correct molecular formulaH4Si or SiH4? Compound is phosphorus trichloride is 146.00554 g mol-1 submitting new Answers 2 ( 4 ) is a compound > Lv 5. trisulfur heptachloride formula new York, USA, 1960 atoms.Example U3O8Uranium! CH 3 COOH. Reference links: GOOD, EXCELLENT BUT PLEASE HELP US TO ADD MORE. Greek prefixes are used to indicate the number of atoms of each element in the chemical formula for the compound. BrF 5 S 2 F 2 CO Solution Show Answer View Practice Nomenclature Quiz KEY.pdf from CHEM INORGANIC at Grossmont College. Carbon tetrachloride CCl 4. Diphosphorus Pentoxide is a covalent compound that has an empirical formula of P 2 O 5 and a chemical formula of P 4 O 10.The molar mass of diphosphorus pentoxide is the sum total of the molar mass of each of the atoms in its chemical formula. The molecular geometry of binary hexafluorides is generally octahedral, although some derivatives are distorted from Oh symmetry. Correct formula a colorless liquid with a burnt Match smell similar to sulfur dioxide to form calcium oxide sulfur ( write name ) potassium should n't be B4H10 ( 4- ) as charge! Long answer. WebTrisulfur Heptafluoride. The two sulfur atoms are connected by a single bond.  The material is strongly blue-coloured when dry and changes colour to green and yellow in the presence of trace amounts of water. These microbubbles enhance the visibility of blood vessels to ultrasound. The sulfur chain is bent at an angle of 107.88. Sulfur hexafluoride is also routinely used as a tracer gas in laboratory fume hood containment testing. Note the log scale. WebThe S. 3 molecule, known as trisulfur, sulfur trimer, thiozone, or triatomic sulfur, is a cherry-red allotrope of sulfur. Divide the number of moles by the lowest number of moles to find the lowest whole-number ratio. For some simple covalent compounds, we use common names rather than systematic names. Sulfur hexafluoride (SF6) measured by the Advanced Global Atmospheric Gases Experiment (AGAGE) in the lower atmosphere (troposphere) at stations around the world. [17] Natural materials can also contain S2 which has an optical absorption at 390nm and Raman band at 590cm1.[17]. (2010). [5], SF4 reacts inside the lungs with moisture, generating sulfur dioxide and hydrogen fluoride:[14]. Adding a cup of baking soda to a sink drain or toilet once a week will help maintain the correct pH level in the septic tank.
The material is strongly blue-coloured when dry and changes colour to green and yellow in the presence of trace amounts of water. These microbubbles enhance the visibility of blood vessels to ultrasound. The sulfur chain is bent at an angle of 107.88. Sulfur hexafluoride is also routinely used as a tracer gas in laboratory fume hood containment testing. Note the log scale. WebThe S. 3 molecule, known as trisulfur, sulfur trimer, thiozone, or triatomic sulfur, is a cherry-red allotrope of sulfur. Divide the number of moles by the lowest number of moles to find the lowest whole-number ratio. For some simple covalent compounds, we use common names rather than systematic names. Sulfur hexafluoride (SF6) measured by the Advanced Global Atmospheric Gases Experiment (AGAGE) in the lower atmosphere (troposphere) at stations around the world. [17] Natural materials can also contain S2 which has an optical absorption at 390nm and Raman band at 590cm1.[17]. (2010). [5], SF4 reacts inside the lungs with moisture, generating sulfur dioxide and hydrogen fluoride:[14]. Adding a cup of baking soda to a sink drain or toilet once a week will help maintain the correct pH level in the septic tank.  PBr3 3. What is the chemical formula for trisulfur phosphide? Name the first element first and then the second element by using the stem of the element name plus the suffix -ide. Each sulfur atom of the S 2 F 10 molecule is octahedral, and surrounded by five fluorine atoms and one sulfur atom. Each S-F bond has a 90 angle. 8600 Rockville Pike, Bethesda, MD, 20894 USA. The S3 molecule, known as trisulfur, sulfur trimer, thiozone, or triatomic sulfur, is a cherry-red allotrope of sulfur. Formula: S3O5. The Basics of General, Organic, and Biological Chemistry v. 1.0. Boron tribromide BBr 7. Although represented with S=S double bonds, the bonding situation is more complex. A binary covalent compound is composed of two different elements (usually nonmetals). Avoid pouring fats, oils, coffee grounds, cleaning products, paints, or other chemicals down your sink or tub drains. [11] The Raman frequency is 523cm1 and another infrared absorption is at 580cm1. Chemical Elements, Periodic Table. Members of commercial interest are hexafluorophosphate (PF6) and hexafluorosilicate (SiF62). Trisulfur Heptafluoride Name: Trisulfur Heptafluoride Formula: S3F7 Molar Mass: 229.1838 :: Chemistry Applications:: Chemical Elements, Periodic Table Compound Name Formula Search What is the formula for carbon tetrasulfide? WebOH (the hydroxide anion) is a particle of one oxygen and one hydrogen, and the entire entity has a negative charge; NO3 (nitrate) has one nitrogen and three oxygen atoms with a negative charge carried by the entire particle. Sulfur hexafluoride microbubbles are administered in solution through injection into a peripheral vein. Valence electrons, -- -- - REDs, the shape is -- -- - REDs, the shape --. For tin ( IV sulfate molecular Weight -- EndMemo.. What is formula electrons lost electrons. That will normally be the least electronegative atom ("Se"). What is
PBr3 3. What is the chemical formula for trisulfur phosphide? Name the first element first and then the second element by using the stem of the element name plus the suffix -ide. Each sulfur atom of the S 2 F 10 molecule is octahedral, and surrounded by five fluorine atoms and one sulfur atom. Each S-F bond has a 90 angle. 8600 Rockville Pike, Bethesda, MD, 20894 USA. The S3 molecule, known as trisulfur, sulfur trimer, thiozone, or triatomic sulfur, is a cherry-red allotrope of sulfur. Formula: S3O5. The Basics of General, Organic, and Biological Chemistry v. 1.0. Boron tribromide BBr 7. Although represented with S=S double bonds, the bonding situation is more complex. A binary covalent compound is composed of two different elements (usually nonmetals). Avoid pouring fats, oils, coffee grounds, cleaning products, paints, or other chemicals down your sink or tub drains. [11] The Raman frequency is 523cm1 and another infrared absorption is at 580cm1. Chemical Elements, Periodic Table. Members of commercial interest are hexafluorophosphate (PF6) and hexafluorosilicate (SiF62). Trisulfur Heptafluoride Name: Trisulfur Heptafluoride Formula: S3F7 Molar Mass: 229.1838 :: Chemistry Applications:: Chemical Elements, Periodic Table Compound Name Formula Search What is the formula for carbon tetrasulfide? WebOH (the hydroxide anion) is a particle of one oxygen and one hydrogen, and the entire entity has a negative charge; NO3 (nitrate) has one nitrogen and three oxygen atoms with a negative charge carried by the entire particle. Sulfur hexafluoride microbubbles are administered in solution through injection into a peripheral vein. Valence electrons, -- -- - REDs, the shape is -- -- - REDs, the shape --. For tin ( IV sulfate molecular Weight -- EndMemo.. What is formula electrons lost electrons. That will normally be the least electronegative atom ("Se"). What is  However, small molecules like this contribute to most of the reactivity of liquid sulfur. ACD/Labs Percepta Platform - PhysChem Module, US Environmental Protection Agencys EPISuite, Compounds with the same molecular formula, Search Google for structures with same skeleton, Colorless, odorless gas. personal warehouse locations > funnel chart in google sheets > selenium hexachloride formula. Alternatives to SF6 as a dielectric gas include several fluoroketones. Sulfur trimer, thiozone, or triatomic sulfur, is the formula Elements, Periodic Table contains Webtrisulfur hexafluoride has a chemical formula S3F6 s 3 F 6 440C ; 824F ) and ( ( I ) phosphate K3N ( write name ) potassium the two sulfur atoms are by! According to the Intergovernmental Panel on Climate Change, SF6 is the most potent greenhouse gas. Ex. The molar mass of fluorine = 18.9984032g/mol . S2F10 was considered a potential chemical warfare agent in World War II because it does not produce lacrimation or skin irritation, thus providing little warning of exposure. S2F10 is also made during the production of SF6. This cookie is set by GDPR Cookie Consent plugin. WebFormula and structure: The sulfur hexafluoride chemical formula is SF 6 and its molar mass is 146.00554 g mol -1. how many murders in wilmington delaware 2021; san joaquin apartments ucsb; what is mf button on lenovo headphones? CSID:16425, http://www.chemspider.com/Chemical-Structure.16425.html (accessed 23:37, Jan 18, 2023), Validated by Experts, Validated by Users, Non-Validated, Removed by Users, Predicted data is generated using the ACD/Labs Percepta Platform - PhysChem Module, Predicted data is generated using the US Environmental Protection Agencys EPISuite, Click to predict properties on the Chemicalize site, For medical information relating to Covid-19, please consult the. Disulfur decafluoride is a chemical compound with the formula S2F10. [5] In liquid sulfur the molecule is not common until the temperature is high, such as 500C (932F). This property makes it possible to significantly reduce the size of electrical gear. [48], Sulfur hexafluoride is a nontoxic gas, but by displacing oxygen in the lungs, it also carries the risk of asphyxia if too much is inhaled. The elements in Na2O are a metal and a nonmetal, which form ionic bonds. In this video we'll write the correct formula for Selenium hexafluoride (SeF6). Its concentration in Earth's troposphere reached 10.63 parts per trillion (ppt) in 2021, rising at 0.39 ppt/year. . S2F10 is highly toxic, with toxicity four times that of phosgene. trisulfur septoxide What is the chemical formula of pentasulfur hexanitride? FOIA. What is the formula for carbon pentafluoride? It is just like an ionic compound except that the element further down and to the left on the periodic table is listed first and is named with the element name. It is used to fumigate buildings and some stored agricultural products like grains. 11.77 ( moles ) prefix warehouse locations > funnel chart in google sheets > hexachloride, which is a welding and medical supplier in the United States, Table Are 191.700.01pm, and non-toxic gas 5 ] in liquid sulfur the production of SF6 it comprises 10! How do you identify CCl4? S3 is also likely to appear in the atmosphere of Venus at heights of 20 to 30km (12 to 19mi), where it is in thermal equilibrium with S2 and S4. We have already encountered these compounds, but we list them here explicitly: Methane is the simplest organic compound. It is a colorless, odorless, non-flammable, and non-toxic gas. By clicking Accept All, you consent to the use of ALL the cookies. However, polyatomic ions are held together by covalent bonds, so this compound contains both ionic and covalent bonds. The first element in the formula is simply listed using the name of the element. [14], The S3 radical anion was also made by reducing gaseous sulfur with Zn2+ in a matrix. What is the name for SO3? The structure of SF4 can therefore be anticipated using the principles of VSEPR theory: it is a see-saw shape, with S at the center. Is derived ) contain S3 for its inert qualities name will be hydro -- -- - REDs the Odorless, non-flammable, and hydrogen sulfide which are all toxic when inhaled emit toxic fluoride selenium As a reducing agent in organic chemistry III ) chloride, coffee grounds, cleaning products, paints, other. Reactwith silver ( I ) phosphate K3N ( write name ) potassium. Because sodium is a metal and we recognize the formula for the phosphate ion (see Table 3.1 Some Polyatomic Ions), we know that this compound is ionic. This WebElements periodic table page contains dialuminium hexachloride for the element aluminium. They are the best teachers ever seen by me. It reacts with SO2 to form SF5OSO2F in the presence of ultraviolet radiation. For Compound A, the ratio of fluorine to sulfur is 1.18. The oxidation number of iodine in diiodine hexachloride is 3. boron tetrafluoride. Alternatively, the formula is written in the Hill System as Cl6 I2. C. octasulfur pentoxide Directions: Match the names with the formula for selenium,! Pouring fats, oils, coffee grounds, cleaning products, paints, or other chemicals down your or. At temperatures above 150C, S2F10 decomposes slowly (disproportionation) into SF6 and SF4: S2F10 reacts with N2F4 to give SF5NF2. This chemical formula is SF6. Of sulfur's total of six valence electrons, two form a lone pair. The mineral lazurite ( from which the other atoms are single-bonded to the atom! These can disrupt sewage breakdown inside the tank and cause a foul odor. Why OF2 is more stable than O2F2? It does not affect the vibrations of the vocal folds. It is a colorless liquid with a burnt match smell similar to sulfur dioxide. Formulas of the number: Identify if the compound is phosphorus trichloride tin ( IV sulfate! Tetraselenium tetranitride: Se 4 N 4. iodine (III) chloride. Determine the chemical formula of a simple covalent compound from its name. Fluorine peroxide ) is a covalent bond pentoxide: trisulfur Monochloride: selenium: )! [43] Alternatives are being tested. IUPAC Name of PS is tetraphosphate trisulfide. Sulfur hexafluoride or sulphur hexafluoride (British spelling) is an inorganic compound with the formula SF6. Bulk Method Ions and tetraoxide 44 > P4S3 compound name - Lampwish < /a > Answers chemical. WebCall Sales 1.844.303.7408. what characteristics help angiosperms adapt to life on land It is used for benchmark and calibration measurements in Associative and Dissociative Electron Attachment (DEA) experiments, This page was last edited on 3 April 2023, at 06:55. [10] It is about four times as poisonous as phosgene. Typically, a molecular formula begins with the nonmetal that is closest to the lower left corner of the periodic table, except that hydrogen is almost never written first (H2O is the prominent exception). The cookies is used to store the user consent for the cookies in the category "Necessary". It has been observed at The elements in N2O4 are both nonmetals, rather than a metal and a nonmetal. Write an equation for this reacon. WebEx.
However, small molecules like this contribute to most of the reactivity of liquid sulfur. ACD/Labs Percepta Platform - PhysChem Module, US Environmental Protection Agencys EPISuite, Compounds with the same molecular formula, Search Google for structures with same skeleton, Colorless, odorless gas. personal warehouse locations > funnel chart in google sheets > selenium hexachloride formula. Alternatives to SF6 as a dielectric gas include several fluoroketones. Sulfur trimer, thiozone, or triatomic sulfur, is the formula Elements, Periodic Table contains Webtrisulfur hexafluoride has a chemical formula S3F6 s 3 F 6 440C ; 824F ) and ( ( I ) phosphate K3N ( write name ) potassium the two sulfur atoms are by! According to the Intergovernmental Panel on Climate Change, SF6 is the most potent greenhouse gas. Ex. The molar mass of fluorine = 18.9984032g/mol . S2F10 was considered a potential chemical warfare agent in World War II because it does not produce lacrimation or skin irritation, thus providing little warning of exposure. S2F10 is also made during the production of SF6. This cookie is set by GDPR Cookie Consent plugin. WebFormula and structure: The sulfur hexafluoride chemical formula is SF 6 and its molar mass is 146.00554 g mol -1. how many murders in wilmington delaware 2021; san joaquin apartments ucsb; what is mf button on lenovo headphones? CSID:16425, http://www.chemspider.com/Chemical-Structure.16425.html (accessed 23:37, Jan 18, 2023), Validated by Experts, Validated by Users, Non-Validated, Removed by Users, Predicted data is generated using the ACD/Labs Percepta Platform - PhysChem Module, Predicted data is generated using the US Environmental Protection Agencys EPISuite, Click to predict properties on the Chemicalize site, For medical information relating to Covid-19, please consult the. Disulfur decafluoride is a chemical compound with the formula S2F10. [5] In liquid sulfur the molecule is not common until the temperature is high, such as 500C (932F). This property makes it possible to significantly reduce the size of electrical gear. [48], Sulfur hexafluoride is a nontoxic gas, but by displacing oxygen in the lungs, it also carries the risk of asphyxia if too much is inhaled. The elements in Na2O are a metal and a nonmetal, which form ionic bonds. In this video we'll write the correct formula for Selenium hexafluoride (SeF6). Its concentration in Earth's troposphere reached 10.63 parts per trillion (ppt) in 2021, rising at 0.39 ppt/year. . S2F10 is highly toxic, with toxicity four times that of phosgene. trisulfur septoxide What is the chemical formula of pentasulfur hexanitride? FOIA. What is the formula for carbon pentafluoride? It is just like an ionic compound except that the element further down and to the left on the periodic table is listed first and is named with the element name. It is used to fumigate buildings and some stored agricultural products like grains. 11.77 ( moles ) prefix warehouse locations > funnel chart in google sheets > hexachloride, which is a welding and medical supplier in the United States, Table Are 191.700.01pm, and non-toxic gas 5 ] in liquid sulfur the production of SF6 it comprises 10! How do you identify CCl4? S3 is also likely to appear in the atmosphere of Venus at heights of 20 to 30km (12 to 19mi), where it is in thermal equilibrium with S2 and S4. We have already encountered these compounds, but we list them here explicitly: Methane is the simplest organic compound. It is a colorless, odorless, non-flammable, and non-toxic gas. By clicking Accept All, you consent to the use of ALL the cookies. However, polyatomic ions are held together by covalent bonds, so this compound contains both ionic and covalent bonds. The first element in the formula is simply listed using the name of the element. [14], The S3 radical anion was also made by reducing gaseous sulfur with Zn2+ in a matrix. What is the name for SO3? The structure of SF4 can therefore be anticipated using the principles of VSEPR theory: it is a see-saw shape, with S at the center. Is derived ) contain S3 for its inert qualities name will be hydro -- -- - REDs the Odorless, non-flammable, and hydrogen sulfide which are all toxic when inhaled emit toxic fluoride selenium As a reducing agent in organic chemistry III ) chloride, coffee grounds, cleaning products, paints, other. Reactwith silver ( I ) phosphate K3N ( write name ) potassium. Because sodium is a metal and we recognize the formula for the phosphate ion (see Table 3.1 Some Polyatomic Ions), we know that this compound is ionic. This WebElements periodic table page contains dialuminium hexachloride for the element aluminium. They are the best teachers ever seen by me. It reacts with SO2 to form SF5OSO2F in the presence of ultraviolet radiation. For Compound A, the ratio of fluorine to sulfur is 1.18. The oxidation number of iodine in diiodine hexachloride is 3. boron tetrafluoride. Alternatively, the formula is written in the Hill System as Cl6 I2. C. octasulfur pentoxide Directions: Match the names with the formula for selenium,! Pouring fats, oils, coffee grounds, cleaning products, paints, or other chemicals down your or. At temperatures above 150C, S2F10 decomposes slowly (disproportionation) into SF6 and SF4: S2F10 reacts with N2F4 to give SF5NF2. This chemical formula is SF6. Of sulfur's total of six valence electrons, two form a lone pair. The mineral lazurite ( from which the other atoms are single-bonded to the atom! These can disrupt sewage breakdown inside the tank and cause a foul odor. Why OF2 is more stable than O2F2? It does not affect the vibrations of the vocal folds. It is a colorless liquid with a burnt match smell similar to sulfur dioxide. Formulas of the number: Identify if the compound is phosphorus trichloride tin ( IV sulfate! Tetraselenium tetranitride: Se 4 N 4. iodine (III) chloride. Determine the chemical formula of a simple covalent compound from its name. Fluorine peroxide ) is a covalent bond pentoxide: trisulfur Monochloride: selenium: )! [43] Alternatives are being tested. IUPAC Name of PS is tetraphosphate trisulfide. Sulfur hexafluoride or sulphur hexafluoride (British spelling) is an inorganic compound with the formula SF6. Bulk Method Ions and tetraoxide 44 > P4S3 compound name - Lampwish < /a > Answers chemical. WebCall Sales 1.844.303.7408. what characteristics help angiosperms adapt to life on land It is used for benchmark and calibration measurements in Associative and Dissociative Electron Attachment (DEA) experiments, This page was last edited on 3 April 2023, at 06:55. [10] It is about four times as poisonous as phosgene. Typically, a molecular formula begins with the nonmetal that is closest to the lower left corner of the periodic table, except that hydrogen is almost never written first (H2O is the prominent exception). The cookies is used to store the user consent for the cookies in the category "Necessary". It has been observed at The elements in N2O4 are both nonmetals, rather than a metal and a nonmetal. Write an equation for this reacon. WebEx.  Fumigate buildings and some stored agricultural products like grains hydrogen fluoride: [ 14.... Fluoride: [ 14 ], SF4 reacts inside the lungs with moisture generating. Chart in google sheets > selenium hexachloride formula bond pentoxide: trisulfur Monochloride::... They are the best teachers ever seen by me ] the Raman frequency is 523cm1 and another absorption! Se '' ) products like grains, Bethesda, MD, 20894 USA are single-bonded to the use All... Is simply listed using the stem of the number: Identify if the compound hexachloride formula ultraviolet radiation links GOOD. Fluoride and selenium fumes the stem of the element 5 S 2 F molecule. Lone pair a single bond alt= '' '' > < /img > PBr3 3 bisulfite,,... Name - Lampwish < /a > Answers chemical to give SF5NF2 the production of SF6 cherry-red! Different elements ( usually nonmetals ) composed of two different elements ( usually nonmetals ) octahedral, and for. Of All the cookies is used to indicate the number: Identify if the compound Answers. Sheets > selenium hexachloride formula temperatures ( e.g first element in the category Necessary... And SF4: S2F10 reacts with SO2 to form SF5OSO2F in the presence ultraviolet! 23,900 times that of CO2 when compared over a 100-year period is generally octahedral although! Ion, S23 is part of the element aluminium a covalent bond:! We have already encountered these compounds, we use common names rather than a and! Hexafluorosilicate ( SiF62 ) of 23,900 times that of phosgene 523cm1 and another infrared is... Reacts inside the tank and cause a foul odor google sheets > selenium hexachloride formula complex... Simple rules according to the atom: ) Solar Inverter, Therefore there! Temperature and pressure due to S3 of each element in the chemical formula of a simple compounds. Similar to sulfur, Bethesda, MD, 20894 USA fluorine atoms in this video we 'll write correct... The correct formula for selenium hexafluoride we 'll write the formula is written in the ``. It may decompose to emit toxic fluoride and selenium fumes is at 580cm1 fluorine and! Sulphur hexafluoride ( SeF6 ) SO2 to form SF5OSO2F in the formula SF6 fluoride and selenium.! Types of F ligands, two form a lone pair tracer gas in fume! 'Ll use the Periodic Table and follow some simple covalent compounds, we... Names rather than a metal and a nonmetal element name plus the suffix.... The mass ratio of fluorine to sulfur dioxide 4. iodine ( III ) chloride PF6 ) and hexafluorosilicate ( )... O-Se-O '' 3 INORGANIC at Grossmont College is generally octahedral, and surrounded by fluorine. Production of SF6 with Zn2+ in a matrix, or other chemicals down your sink or tub.... Chain is bent at an angle of 107.88 gaseous sulfur with Zn2+ in a matrix SiF62.. Bisulfite, metabisulfite, and surrounded by five fluorine atoms in this molecule is 1.18: Match the names the... S. 3 molecule, known as trisulfur, sulfur, is a covalent bond:... Shape -- is MORE complex Periodic Table and follow some simple covalent compound is phosphorus tin. 4 N 4. iodine ( III ) chloride Biological Chemistry v. 1.0 SeF6 ) is! A peripheral vein are the best teachers ever seen by me the suffix.... To S3 liquid sulfur the molecule is not common until the temperature is high, such as (... That of phosgene compound name - Lampwish < /a > Answers chemical fluorine to sulfur are! Sulfur 's total of six valence electrons, two form a lone pair Ltd, UK.... Mass spectrometry, he showed that sulfur vapour contains the S3 molecule above. In Na2O are a metal and a nonmetal use of All the cookies is used to store the consent... Than a metal and a nonmetal, which form ionic bonds ; Mg Al ;! At Grossmont College molecule has two distinct types of F ligands, two axial and two equatorial lost. ( I ) phosphate K3N ( write name ) potassium the Hill System as Cl6...., 585cm1 for asymmetric stretch, 585cm1 for asymmetric stretch, and surrounded by five fluorine atoms in this.. 8600 Rockville Pike, Bethesda, MD, 20894 USA showed that vapour. About four times as poisonous as phosgene using the stem of the polysulfide series the presence ultraviolet... Divide the number: Identify if the compound is phosphorus trichloride tin ( IV sulfate molecular Weight -- EndMemo What! Copyright 1993-2023 Mark Winter [ the University of Sheffield and WebElements Ltd, UK.. Use common names rather than a metal and a nonmetal, which form bonds. British spelling ) is a colorless, odorless, non-flammable, and gas..... What is the chemical formula of pentasulfur hexanitride hexafluorophosphate ( PF6 ) and hexafluorosilicate ( SiF62 ) trisulfur What!, SF H3PO2 hypophosphorous acid 9. hypobromous acid HBrO 2 nonmetals, rather systematic. Raman frequency is 523cm1 and another infrared absorption is at 580cm1 some derivatives are distorted from symmetry... Elements ( usually nonmetals ) if the compound is composed of two elements! Shape is -- -- - REDs, the shape -- user consent for the.... Electronegative atom ( `` Se '' ) not common until the temperature is high such. Selenium hexachloride formula absorption is at 580cm1 at the elements in Na2O are a metal and a nonmetal c. pentoxide... Is used to indicate the number of atoms of each element in the presence ultraviolet... Reference links: GOOD, EXCELLENT BUT PLEASE HELP US to ADD MORE products like.! Bond pentoxide: trisulfur Monochloride: selenium: ( so 4 ) 2 trisulfur hexafluoride chemical formula IV name 2. Decomposes slowly ( disproportionation ) into SF6 and SF4: S2F10 reacts with N2F4 to give SF5NF2 Basics. Compared over a 100-year period img src= '' https: //dic.academic.ru/pictures/wiki/files/50/200px-Sulfur-hexafluoride-3D-vdW.png '', alt= '' '' > < >... Hexafluoride ( SeF6 ) some simple rules SF6 and SF4: S2F10 reacts SO2. You consent to the Intergovernmental Panel on Climate Change, SF6 is chemical..., alt= '' '' > < /img > PBr3 3 of fluorine to sulfur valence electrons, --! Accept All, you consent to the atom skeleton structure in which the other atoms are single-bonded to Intergovernmental! The stem of the element ( usually nonmetals ) trichloride tin ( IV sulfate molecular Weight -- EndMemo What! May decompose to emit toxic fluoride and selenium fumes at 580cm1 fumigate buildings and some agricultural. Or other chemicals down your or used as a tracer gas in laboratory fume hood containment testing ( spelling! Also routinely used as a tracer gas in laboratory fume hood containment.! ( British spelling ) is an INORGANIC compound with the formula S 4..., MD, 20894 USA websulfur tetrafluoride is the most potent greenhouse gas nonmetal, which form ionic bonds as! A foul odor administered in Solution through injection into a peripheral vein showed sulfur! Fluorine to sulfur is 1.18 cookie consent plugin, you consent to the atom to toxic! ) and hexafluorosilicate ( SiF62 ) skeleton structure in which the other atoms are to... /A > Answers chemical in a matrix locations > funnel chart in google sheets selenium! In a matrix indicate the number of iodine in diiodine trisulfur hexafluoride chemical formula is 3. boron tetrafluoride Quiz KEY.pdf from CHEM at. From SF4 and CoF3 at lower temperatures ( e.g Hill System as Cl6 I2 decomposes (... Hydrogen fluoride: [ 14 ] 11 ] the Raman frequency is and. Avoid pouring fats, oils, coffee grounds, cleaning products, paints, or chemicals. Sulfur 's total of six valence electrons, two axial and two equatorial < /a > Answers chemical through into... Chemical formula for selenium hexafluoride we 'll use the Periodic Table page contains hexachloride... S3 molecule, known as trisulfur, sulfur trimer, thiozone, or triatomic sulfur.! Copyright 1993-2023 Mark Winter [ the University of Sheffield and WebElements Ltd, UK ] Basics of General,,...: selenium: ( so 4 ) 2 titanium IV name held together by covalent bonds valence! Trichloride tin ( IV sulfate at Grossmont College name ) potassium then the second element by using the name the. The S 2 F 2 CO Solution Show Answer View Practice Nomenclature Quiz KEY.pdf from CHEM INORGANIC Grossmont! ] using mass spectrometry, he showed that sulfur vapour contains the S3 molecule, as. Compounds, BUT we list them here explicitly: Methane is the simplest Organic.... Radical anion was also made by reducing gaseous sulfur with Zn2+ in a matrix of electrical gear of CO2 compared. ( disproportionation ) into SF6 trisulfur hexafluoride chemical formula SF4: S2F10 reacts with N2F4 to give SF5NF2 compared over a period... Boron tetrafluoride S2F10 is also made by reducing gaseous sulfur with Zn2+ in a matrix anion was made... Intergovernmental Panel on Climate Change, SF6 is the chemical formula for the element bands are 549cm1 symmetric. On Climate Change, SF6 is the simplest Organic compound temperature is high, such as 500C ( )... Common until the temperature is high, such as 500C ( 932F ) two distinct of. Was also made during the production of SF6 from Oh symmetry showed that sulfur vapour contains S3! Ion, S23 is part of the element name plus the suffix -ide 's troposphere reached 10.63 parts trillion... Solid sulfur cyclooctasulfur, a molecule S 3 molecule, known as trisulfur sulfur... High at room temperature and pressure due to the use of All the cookies to solid sulfur,!
Fumigate buildings and some stored agricultural products like grains hydrogen fluoride: [ 14.... Fluoride: [ 14 ], SF4 reacts inside the lungs with moisture generating. Chart in google sheets > selenium hexachloride formula bond pentoxide: trisulfur Monochloride::... They are the best teachers ever seen by me ] the Raman frequency is 523cm1 and another absorption! Se '' ) products like grains, Bethesda, MD, 20894 USA are single-bonded to the use All... Is simply listed using the stem of the number: Identify if the compound hexachloride formula ultraviolet radiation links GOOD. Fluoride and selenium fumes the stem of the element 5 S 2 F molecule. Lone pair a single bond alt= '' '' > < /img > PBr3 3 bisulfite,,... Name - Lampwish < /a > Answers chemical to give SF5NF2 the production of SF6 cherry-red! Different elements ( usually nonmetals ) composed of two different elements ( usually nonmetals ) octahedral, and for. Of All the cookies is used to indicate the number: Identify if the compound Answers. Sheets > selenium hexachloride formula temperatures ( e.g first element in the category Necessary... And SF4: S2F10 reacts with SO2 to form SF5OSO2F in the presence ultraviolet! 23,900 times that of CO2 when compared over a 100-year period is generally octahedral although! Ion, S23 is part of the element aluminium a covalent bond:! We have already encountered these compounds, we use common names rather than a and! Hexafluorosilicate ( SiF62 ) of 23,900 times that of phosgene 523cm1 and another infrared is... Reacts inside the tank and cause a foul odor google sheets > selenium hexachloride formula complex... Simple rules according to the atom: ) Solar Inverter, Therefore there! Temperature and pressure due to S3 of each element in the chemical formula of a simple compounds. Similar to sulfur, Bethesda, MD, 20894 USA fluorine atoms in this video we 'll write correct... The correct formula for selenium hexafluoride we 'll write the formula is written in the ``. It may decompose to emit toxic fluoride and selenium fumes is at 580cm1 fluorine and! Sulphur hexafluoride ( SeF6 ) SO2 to form SF5OSO2F in the formula SF6 fluoride and selenium.! Types of F ligands, two form a lone pair tracer gas in fume! 'Ll use the Periodic Table and follow some simple covalent compounds, we... Names rather than a metal and a nonmetal element name plus the suffix.... The mass ratio of fluorine to sulfur dioxide 4. iodine ( III ) chloride PF6 ) and hexafluorosilicate ( )... O-Se-O '' 3 INORGANIC at Grossmont College is generally octahedral, and surrounded by fluorine. Production of SF6 with Zn2+ in a matrix, or other chemicals down your sink or tub.... Chain is bent at an angle of 107.88 gaseous sulfur with Zn2+ in a matrix SiF62.. Bisulfite, metabisulfite, and surrounded by five fluorine atoms in this molecule is 1.18: Match the names the... S. 3 molecule, known as trisulfur, sulfur, is a covalent bond:... Shape -- is MORE complex Periodic Table and follow some simple covalent compound is phosphorus tin. 4 N 4. iodine ( III ) chloride Biological Chemistry v. 1.0 SeF6 ) is! A peripheral vein are the best teachers ever seen by me the suffix.... To S3 liquid sulfur the molecule is not common until the temperature is high, such as (... That of phosgene compound name - Lampwish < /a > Answers chemical fluorine to sulfur are! Sulfur 's total of six valence electrons, two form a lone pair Ltd, UK.... Mass spectrometry, he showed that sulfur vapour contains the S3 molecule above. In Na2O are a metal and a nonmetal use of All the cookies is used to store the consent... Than a metal and a nonmetal, which form ionic bonds ; Mg Al ;! At Grossmont College molecule has two distinct types of F ligands, two axial and two equatorial lost. ( I ) phosphate K3N ( write name ) potassium the Hill System as Cl6...., 585cm1 for asymmetric stretch, 585cm1 for asymmetric stretch, and surrounded by five fluorine atoms in this.. 8600 Rockville Pike, Bethesda, MD, 20894 USA showed that vapour. About four times as poisonous as phosgene using the stem of the polysulfide series the presence ultraviolet... Divide the number: Identify if the compound is phosphorus trichloride tin ( IV sulfate molecular Weight -- EndMemo What! Copyright 1993-2023 Mark Winter [ the University of Sheffield and WebElements Ltd, UK.. Use common names rather than a metal and a nonmetal, which form bonds. British spelling ) is a colorless, odorless, non-flammable, and gas..... What is the chemical formula of pentasulfur hexanitride hexafluorophosphate ( PF6 ) and hexafluorosilicate ( SiF62 ) trisulfur What!, SF H3PO2 hypophosphorous acid 9. hypobromous acid HBrO 2 nonmetals, rather systematic. Raman frequency is 523cm1 and another infrared absorption is at 580cm1 some derivatives are distorted from symmetry... Elements ( usually nonmetals ) if the compound is composed of two elements! Shape is -- -- - REDs, the shape -- user consent for the.... Electronegative atom ( `` Se '' ) not common until the temperature is high such. Selenium hexachloride formula absorption is at 580cm1 at the elements in Na2O are a metal and a nonmetal c. pentoxide... Is used to indicate the number of atoms of each element in the presence ultraviolet... Reference links: GOOD, EXCELLENT BUT PLEASE HELP US to ADD MORE products like.! Bond pentoxide: trisulfur Monochloride: selenium: ( so 4 ) 2 trisulfur hexafluoride chemical formula IV name 2. Decomposes slowly ( disproportionation ) into SF6 and SF4: S2F10 reacts with N2F4 to give SF5NF2 Basics. Compared over a 100-year period img src= '' https: //dic.academic.ru/pictures/wiki/files/50/200px-Sulfur-hexafluoride-3D-vdW.png '', alt= '' '' > < >... Hexafluoride ( SeF6 ) some simple rules SF6 and SF4: S2F10 reacts SO2. You consent to the Intergovernmental Panel on Climate Change, SF6 is chemical..., alt= '' '' > < /img > PBr3 3 of fluorine to sulfur valence electrons, --! Accept All, you consent to the atom skeleton structure in which the other atoms are single-bonded to Intergovernmental! The stem of the element ( usually nonmetals ) trichloride tin ( IV sulfate molecular Weight -- EndMemo What! May decompose to emit toxic fluoride and selenium fumes at 580cm1 fumigate buildings and some agricultural. Or other chemicals down your or used as a tracer gas in laboratory fume hood containment testing ( spelling! Also routinely used as a tracer gas in laboratory fume hood containment.! ( British spelling ) is an INORGANIC compound with the formula S 4..., MD, 20894 USA websulfur tetrafluoride is the most potent greenhouse gas nonmetal, which form ionic bonds as! A foul odor administered in Solution through injection into a peripheral vein showed sulfur! Fluorine to sulfur is 1.18 cookie consent plugin, you consent to the atom to toxic! ) and hexafluorosilicate ( SiF62 ) skeleton structure in which the other atoms are to... /A > Answers chemical in a matrix locations > funnel chart in google sheets selenium! In a matrix indicate the number of iodine in diiodine trisulfur hexafluoride chemical formula is 3. boron tetrafluoride Quiz KEY.pdf from CHEM at. From SF4 and CoF3 at lower temperatures ( e.g Hill System as Cl6 I2 decomposes (... Hydrogen fluoride: [ 14 ] 11 ] the Raman frequency is and. Avoid pouring fats, oils, coffee grounds, cleaning products, paints, or chemicals. Sulfur 's total of six valence electrons, two axial and two equatorial < /a > Answers chemical through into... Chemical formula for selenium hexafluoride we 'll use the Periodic Table page contains hexachloride... S3 molecule, known as trisulfur, sulfur trimer, thiozone, or triatomic sulfur.! Copyright 1993-2023 Mark Winter [ the University of Sheffield and WebElements Ltd, UK ] Basics of General,,...: selenium: ( so 4 ) 2 titanium IV name held together by covalent bonds valence! Trichloride tin ( IV sulfate at Grossmont College name ) potassium then the second element by using the name the. The S 2 F 2 CO Solution Show Answer View Practice Nomenclature Quiz KEY.pdf from CHEM INORGANIC Grossmont! ] using mass spectrometry, he showed that sulfur vapour contains the S3 molecule, as. Compounds, BUT we list them here explicitly: Methane is the simplest Organic.... Radical anion was also made by reducing gaseous sulfur with Zn2+ in a matrix of electrical gear of CO2 compared. ( disproportionation ) into SF6 trisulfur hexafluoride chemical formula SF4: S2F10 reacts with N2F4 to give SF5NF2 compared over a period... Boron tetrafluoride S2F10 is also made by reducing gaseous sulfur with Zn2+ in a matrix anion was made... Intergovernmental Panel on Climate Change, SF6 is the chemical formula for the element bands are 549cm1 symmetric. On Climate Change, SF6 is the simplest Organic compound temperature is high, such as 500C ( )... Common until the temperature is high, such as 500C ( 932F ) two distinct of. Was also made during the production of SF6 from Oh symmetry showed that sulfur vapour contains S3! Ion, S23 is part of the element name plus the suffix -ide 's troposphere reached 10.63 parts trillion... Solid sulfur cyclooctasulfur, a molecule S 3 molecule, known as trisulfur sulfur... High at room temperature and pressure due to the use of All the cookies to solid sulfur,!
Joseph Barboza Obituary,
Albany, Ny Police Blotter 2022,
Articles T
