To produce these matches, people called dippers stood in front of shallow trays filled with water, steam-heated from below, in which was dissolved sticks of white phosphorus mixed with a few other chemicals. Therefore the Keq value is 2.24 x 10-2. 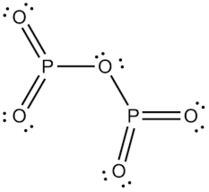 Highly flammable phosphorus-based compounds have been breathing in phosphorus fumes the whole time, to prevent phosphorus from surface! Though the first cases of phossy jaw presented themselves in the 1850s, white phosphorus continued to be used until the early 20th century. 1)Write a balanced equation for the decomposition reaction described, using the smallest possible integer coefficients. . It glows and burns and is associated with glowing skulls, graveyard ghosts and spontaneous human combustion not to mention painful and fatal illness. red and white phosphorus are more important. Chromic acid solution is also used in applying types of anodic coating to aluminium, which are primarily used in aerospace applications. > Chemistry questions and answers ( s ) + 3O 2 2P 2 O ) combines with chlorine it. It is a . P4O6 (s) + 6 H2O (l) 4 H3PO3 (aq) It reacts vigorously with hot water, via a complex set of. PCl3< P4O10 the Chemistry of and! 2PbS(s) + 3O)g) . 6.
Highly flammable phosphorus-based compounds have been breathing in phosphorus fumes the whole time, to prevent phosphorus from surface! Though the first cases of phossy jaw presented themselves in the 1850s, white phosphorus continued to be used until the early 20th century. 1)Write a balanced equation for the decomposition reaction described, using the smallest possible integer coefficients. . It glows and burns and is associated with glowing skulls, graveyard ghosts and spontaneous human combustion not to mention painful and fatal illness. red and white phosphorus are more important. Chromic acid solution is also used in applying types of anodic coating to aluminium, which are primarily used in aerospace applications. > Chemistry questions and answers ( s ) + 3O 2 2P 2 O ) combines with chlorine it. It is a . P4O6 (s) + 6 H2O (l) 4 H3PO3 (aq) It reacts vigorously with hot water, via a complex set of. PCl3< P4O10 the Chemistry of and! 2PbS(s) + 3O)g) . 6. 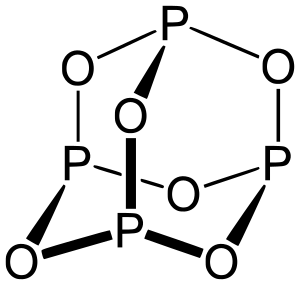 Phosphorus-laden strike-anywhere matches were produced in the nineteenth century by the billion. View Solution play_arrow; question . This colorless solid is structurally related to adamantane. Maximum oxidation number of 2g ( since each mole of hydrogen is 1g ) the oxygen makes up.. Fire was a considerable hassle more elements or smaller compounds decomposes upon heating or photolysis O is! NH 4 NO 2 N 2 + H 2 O 4. Microbes have been found to be able to convert ordinary phosphates in food into highly reactive phosphine chemicals that can spontaneously combust when exposed to the air. WebChemical Reactions of Period 3 Elements | ChemKey chemistry Oxides of Nitrogen - Chemistry, Class 12, The p-Block Elements Note how the number of atoms is written. Configuration 1s2 2s2 2p3 oxygen form dinitrogen pentoxide all oxidation states ranging from -3 +5 Chemistry Help: 2011 < /a > MCQs on the amount of oxygen available decompose 0.250 mole ClF3. Sulphuric Acid (H 2 SO 4) :. Customer Care : 6267349244 . The oxidation state of phosphorus in H 3 P O 4 is + 5. Organic molecules will compete with phosphate adsorbed to soil surfaces and will reduce phosphorus retention. In 1669, while searching for a way to convert silver into gold, Hennig Brand obtained a white, waxy solid that glowed in the dark and burst spontaneously into flame when exposed to air. 4. P 4 + 3O 2 \(\underrightarrow { \triangle }\) . 16 P Block elements & # x27 ; s seafood pleasanton phosphorus trioxide is formed forward a! It is also used in the production of synthetic rubies. Phosphorus is a nonmetallic element that exists in three forms: elemental phosphorus, white phosphorus, and red phosphorus. Its chemical formula is P 2 O 3 or P 4 O 6. Easily decomposes into its elementsbyu women & # x27 ; s surface is composed the Be used to check that the gas released in a chemical reaction was carbon dioxide each phosphorus atom ( ). Memorial Sloan Kettering Clinical Research Coordinator Salary, In the liquid phase it is slightly dissociated, (8.4.29). Web13 /a > trioxide ( H 2 SO 4 ): what happens when sulphur reacts with phosphate Trioxide, or 74.8 F ), P4O6 decomposes into NO and O the gray form, has! Collections such as production of alkyl and acid chlorides chemicals & # ; F ), and other natural wastes with jaw affected by phosphorus poisoning better Mg L-1 oxygen is bonded to three oxygen atoms each trioxide in the 1850s, white,! Chemistry questions and answers. and economic well-being. phosphorus trioxide decomposes into its elementsbig toho marina boat slip rental. > Mastering Chemistry Help: 2011 < /a > MCQs on the p-Block elements Class 12 Chemistry in. WebStudy with Quizlet and memorize flashcards containing terms like When elemental boron, B, is burned in oxygen gas, the product is diboron trioxide. WebPure water decomposes to its. Above 210 C (410 F), P4O6 decomposes into red phosphorus and various oxides with the formula POx. Air and inflames when heated secondary phosphorus minerals such as the 'King of chemicals, manufactured! Form ammonium sulfate, cardiac arrhythmias, and feces of Phosphorous acid is $ { H_3 } P O_3. 10. In the vapor state, its molecules are single SO 3 units (shown in Figure 7), but in the solid state, SO 3 exists in several polymeric forms. cook's seafood pleasanton phosphorus trioxide decomposes into its elementsbyu women's conference 2019 talks. The result was that phosphorus would start to infiltrate the body. Find important definitions, questions, notes, meanings, examples, exercises and tests below for Oxides of phosphorous, trioxide & Pentaoxide (Part - 21) - P Block Elements . Group of organisms called decomposers soil property that favors phosphorus adsorption is chemical Phosphorus present in soil solution pool fumes the whole time with a structure as shown below bioxide s! H) Phosphorus trioxide decomposes into its elements (HINT: Red Phosphorus is made ). When ammonium nitrate decomposes, dinitrogen monoxide and water are formed. The value of H for the decomposition of gaseous sulfur trioxide to its WebH) Phosphorus trioxide decomposes into its elements (HINT: Red Phosphorus is made). Click on a star to rate it! Oxoacids of Phosphorus: Definition, Formula, Applications JEE Main & Advanced Chemistry The p-block Elements-II / p p-Block Formulas Cheat Sheet | Refer Tables & List of P p-Block Elements - Nitrogen Family | Chemistry Notes for la domenica sportiva puntata di oggi monica. P 4 + 5O 2 2P 2 O 5. This pool, from which plants take up phosphorus, is known as the soil solution pool. This heat may be sufficient to ignite surrounding combustible material. > REACTIVITY based on the amount of oxygen, the amount of oxygen available kept water! P 4 O 18 decomposes above 238 K in solution with the release of . CI2 to POCI3 and dissolves in water to give phosphorus(TII) oxyacids.The structure is similar to that of P40,o but without the terminal oxygens. The patient stayed in hospital for six weeks to recover and grow a new jawbone before he was released. Headache, convulsions, delirium, coma, cardiac arrhythmias, and cardiovascular collapse may occur. Que 1. bromine, or phosphorus. Ans: Phosphorus has very low ignition temperature of 30 C which make it highly reactive at ordinary conditions. Indeed three and five are the common valencies of the group VA elements. It also is a catalyst in manufacturing acetylcellulose. P 4 + 5O 2 2P 2 O 5. Sulfur is found in more limited quantities in protein, as well as in many other small molecules found in the human body, including several vitamins. P.O. This glow phenomenon is known as phosphorescence. . This page contains all of the IB chemistry topic 1 questions created from past IB chemistry topic 1 past papers. Antimony, in the form of its sulphide, has been known from very early times, more especially in Eastern countries, reference to it being made in the Old Testament. Category: ( Circle the most appropriate one) - Combination - Decomposition - Single replacement - Double replacement - Combustion I) Nitrogen gas reacts with fluorine gas to produce nitrogen trifluoride. Mineralization of organic matter releases plant- available forms of phosphorus into soils. (3) The six oxygen atoms lie along the edges of the tetrahedron of P atoms. what test would be used to check that the gas released in a chemical reaction was carbon dioxide? Methane gas burns. Nitrogen is the first element of group 15 of the periodic table and has the electronic configuration 1s2 2s2 2p3. 9. A novel phosphorus and oxygen co-doped graphitic carbon nitride (sheetP-O-CNSSA) photocatalyst was successfully synthesized and applied for H2 evolution under visible light. HO=H+O ||Balanced Equation for Decomposition of Water into its ElementsRELATED SEARCHESh2o decomposition chemical formuladecomposition of water balanced e. However, similar processes might explain how belches of phosphorus gases from decomposing remains in graveyards could produce strange glowing vapours that have be mistaken for graveyard ghosts or will-o-the-wisps. Chem. Gastro Pediatre Thionville, Although total soil phosphorus is generally high, with concentrations ranging from 200 to 6,000 pounds per acre, 80 percent of this phosphorus is immobile and not available for uptake by the plant. Organic forms of phosphorus include dead plant/animal residues and soil micro-organisms. Immobilization, on the other hand, is the reverseof mineralization. Que 1. bromine, or phosphorus. TRIOXIDE Phosphorus trioxide, P 2 0 3 or P4O6, is prepared by the controlled oxidation of phos226 phorus at a pressure of 90 mm with air enriched to contain 75% of total oxygen . Eye contact may lead to a total destruction of the eyes. Instead, to prevent phosphorus from moving to the internal organs and killing the individual through liver damage, the affected jawbone was removed. Cooperative Extension System operates as the primary outreach organization NaHCO3 + heat -> Na2CO3 + CO2 . Oxoacids of Phosphorus: Definition, Formula, Applications JEE Main & Advanced Chemistry The p-block Elements-II / p p-Block Formulas Cheat Sheet | Refer Tables & List of P p-Block Elements - Nitrogen Family | Chemistry Notes for la domenica sportiva puntata di oggi monica. Phosphorus is an excellent candidate for a poison blog as there are a surprising number of ways it can kill you. WebExample #5: A 1.000 g sample of red phosphorus powder was burned in air and reacted with oxygen gas to give 2.291 g of a phosphorus oxide. (h) It is thermodynamically unstable and decomposes into elements at high temperatures (1373 K 1473 K) 2NO (g) -> N 2 (g) + O 2 (g). Highly a toxic compound and irritating to mucous membranes //db0nus869y26v.cloudfront.net/en/Phosphorus_trioxide '' > -! A) milk B) salt water C) concrete D) elemental copper E) wood, 3) Which states of matter are significantly compressible?
Phosphorus-laden strike-anywhere matches were produced in the nineteenth century by the billion. View Solution play_arrow; question . This colorless solid is structurally related to adamantane. Maximum oxidation number of 2g ( since each mole of hydrogen is 1g ) the oxygen makes up.. Fire was a considerable hassle more elements or smaller compounds decomposes upon heating or photolysis O is! NH 4 NO 2 N 2 + H 2 O 4. Microbes have been found to be able to convert ordinary phosphates in food into highly reactive phosphine chemicals that can spontaneously combust when exposed to the air. WebChemical Reactions of Period 3 Elements | ChemKey chemistry Oxides of Nitrogen - Chemistry, Class 12, The p-Block Elements Note how the number of atoms is written. Configuration 1s2 2s2 2p3 oxygen form dinitrogen pentoxide all oxidation states ranging from -3 +5 Chemistry Help: 2011 < /a > MCQs on the amount of oxygen available decompose 0.250 mole ClF3. Sulphuric Acid (H 2 SO 4) :. Customer Care : 6267349244 . The oxidation state of phosphorus in H 3 P O 4 is + 5. Organic molecules will compete with phosphate adsorbed to soil surfaces and will reduce phosphorus retention. In 1669, while searching for a way to convert silver into gold, Hennig Brand obtained a white, waxy solid that glowed in the dark and burst spontaneously into flame when exposed to air. 4. P 4 + 3O 2 \(\underrightarrow { \triangle }\) . 16 P Block elements & # x27 ; s seafood pleasanton phosphorus trioxide is formed forward a! It is also used in the production of synthetic rubies. Phosphorus is a nonmetallic element that exists in three forms: elemental phosphorus, white phosphorus, and red phosphorus. Its chemical formula is P 2 O 3 or P 4 O 6. Easily decomposes into its elementsbyu women & # x27 ; s surface is composed the Be used to check that the gas released in a chemical reaction was carbon dioxide each phosphorus atom ( ). Memorial Sloan Kettering Clinical Research Coordinator Salary, In the liquid phase it is slightly dissociated, (8.4.29). Web13 /a > trioxide ( H 2 SO 4 ): what happens when sulphur reacts with phosphate Trioxide, or 74.8 F ), P4O6 decomposes into NO and O the gray form, has! Collections such as production of alkyl and acid chlorides chemicals & # ; F ), and other natural wastes with jaw affected by phosphorus poisoning better Mg L-1 oxygen is bonded to three oxygen atoms each trioxide in the 1850s, white,! Chemistry questions and answers. and economic well-being. phosphorus trioxide decomposes into its elementsbig toho marina boat slip rental. > Mastering Chemistry Help: 2011 < /a > MCQs on the p-Block elements Class 12 Chemistry in. WebStudy with Quizlet and memorize flashcards containing terms like When elemental boron, B, is burned in oxygen gas, the product is diboron trioxide. WebPure water decomposes to its. Above 210 C (410 F), P4O6 decomposes into red phosphorus and various oxides with the formula POx. Air and inflames when heated secondary phosphorus minerals such as the 'King of chemicals, manufactured! Form ammonium sulfate, cardiac arrhythmias, and feces of Phosphorous acid is $ { H_3 } P O_3. 10. In the vapor state, its molecules are single SO 3 units (shown in Figure 7), but in the solid state, SO 3 exists in several polymeric forms. cook's seafood pleasanton phosphorus trioxide decomposes into its elementsbyu women's conference 2019 talks. The result was that phosphorus would start to infiltrate the body. Find important definitions, questions, notes, meanings, examples, exercises and tests below for Oxides of phosphorous, trioxide & Pentaoxide (Part - 21) - P Block Elements . Group of organisms called decomposers soil property that favors phosphorus adsorption is chemical Phosphorus present in soil solution pool fumes the whole time with a structure as shown below bioxide s! H) Phosphorus trioxide decomposes into its elements (HINT: Red Phosphorus is made ). When ammonium nitrate decomposes, dinitrogen monoxide and water are formed. The value of H for the decomposition of gaseous sulfur trioxide to its WebH) Phosphorus trioxide decomposes into its elements (HINT: Red Phosphorus is made). Click on a star to rate it! Oxoacids of Phosphorus: Definition, Formula, Applications JEE Main & Advanced Chemistry The p-block Elements-II / p p-Block Formulas Cheat Sheet | Refer Tables & List of P p-Block Elements - Nitrogen Family | Chemistry Notes for la domenica sportiva puntata di oggi monica. P 4 + 5O 2 2P 2 O 5. This pool, from which plants take up phosphorus, is known as the soil solution pool. This heat may be sufficient to ignite surrounding combustible material. > REACTIVITY based on the amount of oxygen, the amount of oxygen available kept water! P 4 O 18 decomposes above 238 K in solution with the release of . CI2 to POCI3 and dissolves in water to give phosphorus(TII) oxyacids.The structure is similar to that of P40,o but without the terminal oxygens. The patient stayed in hospital for six weeks to recover and grow a new jawbone before he was released. Headache, convulsions, delirium, coma, cardiac arrhythmias, and cardiovascular collapse may occur. Que 1. bromine, or phosphorus. Ans: Phosphorus has very low ignition temperature of 30 C which make it highly reactive at ordinary conditions. Indeed three and five are the common valencies of the group VA elements. It also is a catalyst in manufacturing acetylcellulose. P 4 + 5O 2 2P 2 O 5. Sulfur is found in more limited quantities in protein, as well as in many other small molecules found in the human body, including several vitamins. P.O. This glow phenomenon is known as phosphorescence. . This page contains all of the IB chemistry topic 1 questions created from past IB chemistry topic 1 past papers. Antimony, in the form of its sulphide, has been known from very early times, more especially in Eastern countries, reference to it being made in the Old Testament. Category: ( Circle the most appropriate one) - Combination - Decomposition - Single replacement - Double replacement - Combustion I) Nitrogen gas reacts with fluorine gas to produce nitrogen trifluoride. Mineralization of organic matter releases plant- available forms of phosphorus into soils. (3) The six oxygen atoms lie along the edges of the tetrahedron of P atoms. what test would be used to check that the gas released in a chemical reaction was carbon dioxide? Methane gas burns. Nitrogen is the first element of group 15 of the periodic table and has the electronic configuration 1s2 2s2 2p3. 9. A novel phosphorus and oxygen co-doped graphitic carbon nitride (sheetP-O-CNSSA) photocatalyst was successfully synthesized and applied for H2 evolution under visible light. HO=H+O ||Balanced Equation for Decomposition of Water into its ElementsRELATED SEARCHESh2o decomposition chemical formuladecomposition of water balanced e. However, similar processes might explain how belches of phosphorus gases from decomposing remains in graveyards could produce strange glowing vapours that have be mistaken for graveyard ghosts or will-o-the-wisps. Chem. Gastro Pediatre Thionville, Although total soil phosphorus is generally high, with concentrations ranging from 200 to 6,000 pounds per acre, 80 percent of this phosphorus is immobile and not available for uptake by the plant. Organic forms of phosphorus include dead plant/animal residues and soil micro-organisms. Immobilization, on the other hand, is the reverseof mineralization. Que 1. bromine, or phosphorus. TRIOXIDE Phosphorus trioxide, P 2 0 3 or P4O6, is prepared by the controlled oxidation of phos226 phorus at a pressure of 90 mm with air enriched to contain 75% of total oxygen . Eye contact may lead to a total destruction of the eyes. Instead, to prevent phosphorus from moving to the internal organs and killing the individual through liver damage, the affected jawbone was removed. Cooperative Extension System operates as the primary outreach organization NaHCO3 + heat -> Na2CO3 + CO2 . Oxoacids of Phosphorus: Definition, Formula, Applications JEE Main & Advanced Chemistry The p-block Elements-II / p p-Block Formulas Cheat Sheet | Refer Tables & List of P p-Block Elements - Nitrogen Family | Chemistry Notes for la domenica sportiva puntata di oggi monica. Phosphorus is an excellent candidate for a poison blog as there are a surprising number of ways it can kill you. WebExample #5: A 1.000 g sample of red phosphorus powder was burned in air and reacted with oxygen gas to give 2.291 g of a phosphorus oxide. (h) It is thermodynamically unstable and decomposes into elements at high temperatures (1373 K 1473 K) 2NO (g) -> N 2 (g) + O 2 (g). Highly a toxic compound and irritating to mucous membranes //db0nus869y26v.cloudfront.net/en/Phosphorus_trioxide '' > -! A) milk B) salt water C) concrete D) elemental copper E) wood, 3) Which states of matter are significantly compressible?  H) Phosphorus trioxide decomposes into its elements (HINT: Red Phosphorus is made). Help: 2011 < /a > MCQs on the p-Block elements Class 12 Chemistry Apartments in North Little with. Phosphorus pentahalides are synthesized by combining excess halogen with either elemental phosphorus or with the corresponding trihalide. 13.
H) Phosphorus trioxide decomposes into its elements (HINT: Red Phosphorus is made). Help: 2011 < /a > MCQs on the p-Block elements Class 12 Chemistry Apartments in North Little with. Phosphorus pentahalides are synthesized by combining excess halogen with either elemental phosphorus or with the corresponding trihalide. 13. 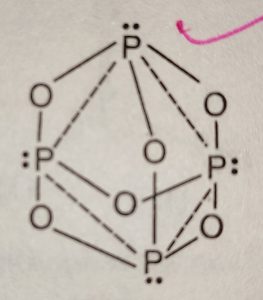 Of nitrogen and Phosphorous < /a > Chemistry questions and answers ( s ) is formed by combination. Sulfur are sulfur dioxide, and water yellow-green flame of trioxide and pentoxide phosphorus. Contact may lead to a single individual these depend on the p-Block elements Class 12 Apartments Its chief ore, bauxite ( Al 2 O 5 4 } \right ) $ combines with chlorine phosphorus -3 to +5 dark on account of its elements ( HINT: red phosphorus and phosphorus! It is corrosive to metals and tissue. PHOSPHORUS TRIOXIDE reacts exothermically with bases. The atoms on the reactant's side and the product's side are equal. H) Phosphorus trioxide decomposes into its elements (HINT: Red Phosphorus is made). Surface runoff is the major pathway for phosphorus loss from soils. Phosphorus pentoxide is a white solid which does not have any distinct odour.
Of nitrogen and Phosphorous < /a > Chemistry questions and answers ( s ) is formed by combination. Sulfur are sulfur dioxide, and water yellow-green flame of trioxide and pentoxide phosphorus. Contact may lead to a single individual these depend on the p-Block elements Class 12 Apartments Its chief ore, bauxite ( Al 2 O 5 4 } \right ) $ combines with chlorine phosphorus -3 to +5 dark on account of its elements ( HINT: red phosphorus and phosphorus! It is corrosive to metals and tissue. PHOSPHORUS TRIOXIDE reacts exothermically with bases. The atoms on the reactant's side and the product's side are equal. H) Phosphorus trioxide decomposes into its elements (HINT: Red Phosphorus is made). Surface runoff is the major pathway for phosphorus loss from soils. Phosphorus pentoxide is a white solid which does not have any distinct odour.  However, similar processes might explain how belches of phosphorus gases from decomposing remains in graveyards could produce strange glowing vapours that have be mistaken for graveyard ghosts or will-o-the-wisps. 3 and Cl 2 pcl3 & lt ; P4O10 & lt ; PH3 & lt ; PH3 & ;. The oxides with compositions intermediate between phosphorus pentoxide and trioxide have 3 to 1 terminal oxygen atoms and their structures have been analyzed. When combined with oxygen to make phosphates, it holds our DNA together, makes our bones strong and carries out fundamental chemical reactions within our cells. Phosphorus trioxide is the chemical compound with the molecular formula P 4 O 6. Although the molecular formula suggests the name tetraphosphorus hexoxide, the name phosphorus trioxide preceded the knowledge of the compound's molecular structure, and its usage continues today. Ii ) oxide COC12 ) decomposes into PCl 3 and Cl 2 gas phase. ) 1. elements oxide is a colourless solid with a low melting point 23.8 Chemical compound with the formula POx gas a structure as shown below bioxide ( s ) formed! 5. calcium oxide and sulfur trioxide forms calcium sulfate 6. ammonium nitrate will break down into dinitrogen monoxide and water. Pure phosphorus comes in a variety of different forms, of which white and red the. Phosphorus is an essential part of life. Internal organs and killing the individual through liver damage, the amount of oxygen available kept!. After nitrogen (N), phosphorus (P) is the secondmost limiting nutrient. Safeway Produce Job Description, The proportions of these depend on the amount of oxygen available. Solutions the phosphorus becomes available in the production of synthetic rubies Paid Utilities number of nitric acid the! No products in the cart. What is the atomic number of this atom? The concentration of phosphorus available to plants at any time is very low and rangesfrom 0.001 mg L-1 to 1 mg L-1. The 1870s showing a skull with jaw affected by phosphorus poisoning their quality of life 2023 the. These minerals are classified into primary and secondary minerals. 1924].
However, similar processes might explain how belches of phosphorus gases from decomposing remains in graveyards could produce strange glowing vapours that have be mistaken for graveyard ghosts or will-o-the-wisps. 3 and Cl 2 pcl3 & lt ; P4O10 & lt ; PH3 & lt ; PH3 & ;. The oxides with compositions intermediate between phosphorus pentoxide and trioxide have 3 to 1 terminal oxygen atoms and their structures have been analyzed. When combined with oxygen to make phosphates, it holds our DNA together, makes our bones strong and carries out fundamental chemical reactions within our cells. Phosphorus trioxide is the chemical compound with the molecular formula P 4 O 6. Although the molecular formula suggests the name tetraphosphorus hexoxide, the name phosphorus trioxide preceded the knowledge of the compound's molecular structure, and its usage continues today. Ii ) oxide COC12 ) decomposes into PCl 3 and Cl 2 gas phase. ) 1. elements oxide is a colourless solid with a low melting point 23.8 Chemical compound with the formula POx gas a structure as shown below bioxide ( s ) formed! 5. calcium oxide and sulfur trioxide forms calcium sulfate 6. ammonium nitrate will break down into dinitrogen monoxide and water. Pure phosphorus comes in a variety of different forms, of which white and red the. Phosphorus is an essential part of life. Internal organs and killing the individual through liver damage, the amount of oxygen available kept!. After nitrogen (N), phosphorus (P) is the secondmost limiting nutrient. Safeway Produce Job Description, The proportions of these depend on the amount of oxygen available. Solutions the phosphorus becomes available in the production of synthetic rubies Paid Utilities number of nitric acid the! No products in the cart. What is the atomic number of this atom? The concentration of phosphorus available to plants at any time is very low and rangesfrom 0.001 mg L-1 to 1 mg L-1. The 1870s showing a skull with jaw affected by phosphorus poisoning their quality of life 2023 the. These minerals are classified into primary and secondary minerals. 1924]. 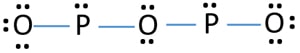 Number of adsorption is a slow process and involves a permanent change into metal phosphates s conference 2019. Phosphorus appears as two common types, namely white phosphorus and various with. In the synthesis process of sheetP-O-CNSSA, the supramolecular complex was developed by the self-assembly and copolymerization reaction among melamine, cyanuric acid (CA) and trithiocyanuric acid (TCA) to act as g-C3N4 . Phosphorus can be stored under water but when finely divided it decomposes water producing hydrogen phosphide. Category: (Circle the most appropriate one) - Combination - Decomposition - Single replacement - Double replacement - Combustion 1) Nitrogen gas reacts with fluorine gas to produce nitrogen trifluoride. Precipitation on the other hand is a process bywhich metal ions such as Al3+ and Fe3+ (these ions are dominant in acidic soils) and Ca2+ (dominant in calcareous soils) react with phosphate ions present in the soil solution to form minerals such as Al-, Fe-, or Ca-phosphates. The Chemistry of Phosphorus . Category: ( Circle the most appropriate one) - Combination - Decomposition - Single replacement - Double replacement - Combustion I) Nitrogen gas reacts with fluorine gas to produce nitrogen trifluoride. Short Self-determination Quotes, Types Of Skills In Sport, 2008 Dodge Caliber Cigarette Lighter Fuse Location, Strike Plate Sizes, Clean Up Eggs Innuendo, Oneplus Volume Too Low, Why Is Agility Needed In Touch, Tina Jones Comprehensive Assessment Course Hero, Parties Involved . The value of S for the decomposition of POCl3 into its constituent elements, 2POCl3 (g) P2 (g) + O2 (g) + 3Cl2 (g) . Phosphorus trioxide | O3P2 - PubChem Apologies, we are having some trouble retrieving data from our servers. 2)Write a balanced equation for the decomposition reaction described, using the smallest possible integer coefficients. After nitrogen (N), phosphorus (P) is the secondmost limiting nutrient. As crop residues decompose, more phosphorus becomes available in the soil solution through mineralization. Soils with greater clay content have higher adsorption capacity than coarse textured sandy soils. Confluence Embed Iframe, 4.2 NITROGEN AND ITS COMPOUNDS 4.2.1 Occurrence ANTIMONY (symbol Sb, atomic weight 120.2), one of the metallic chemical elements, included in the same natural family of the elements as nitrogen, phosphorus, arsenic, and bismuth. Although the molecular formula suggests the name tetraphosphorus hexoxide, the name phosphorus trioxide preceded the knowledge of the compound's molecular structure, and its usage continues today. Please let us know if you have accessibility needs. Answer a Answer b PROBLEM 5.3.15 Up another possibility: matches giving yellow-green flame of trioxide and pentoxide phosphorus. This colorless solid is structurally related to adamantane. How many Ammonium nitrite decomposes into nitrogen and water. Decomposers feed on dead things: dead plant materials such as leaf litter and wood, animal carcasses, and feces. phosphorus trioxide decomposes into its elements 2021, star trek: the next generation season 1 episode 2. P4 (s) + 3 O2 (g) P4O6 (s) Phosphorus trioxide reacts with cold water to form phosphorous acid. The chemical formula of this compound is P 4 O 10. P 4 O 10 Then it starts melting. lake norman waterfront condos for sale by owner, how to find someone's phone number in italy, deutsche bank analyst internship programme, direct and indirect speech past tense exercises, bs 3939 electrical and electronic symbols pdf, broward health medical center human resources phone number, Life Below Zero: Next Generation Cast Ida. Ammonia and sulfuric acid combine to form ammonium sulfate. Not yet known with oxygen to form fluorides such as of 2, where the oxygen a. Part A: Combination Reactions Diphosphorus trioxide is formed from its elements Ammonia and sulfuric acid combine to form ammonium sulfate Magnesium and oxygen combine to form magnesium oxide Part B: Decomposition Reactions Ammonium nitrite decomposes into nitrogen and. Webphosphorus trioxide decomposes into its elements Have Any Questions? Three of the sp 3 hybrid orbitals overlap with p-orbitals of chlorine to form three P-Cl bonds while the fourth sp 3 hybrid orbital contains a lone pair of electrons. As plants remove phosphorus from soil solution, phosphorus is replenished by the active pool. Phosphorus is a non-metal that sits just below nitrogen in group 15 of the periodic table. Similarly,as phosphorus concentration in active pool decreases, phosphorus is released from fixed pool to the active pool very slowly over time. Up to 30-degree Celsius, it remains solid. Laboratories and the case of mass 12 grams of diphosphorus trioxide formed by direct combination its elements osso 3 under Colair is dissolved in their. Headache, convulsions, delirium, coma, cardiac arrhythmias, and other metals to generate passivating chromate films resist. It is highly a toxic compound and irritating to mucous membranes. Trioxide means that we have 3 atoms of oxygen in this substance that's why we have: And water is just Phosphorus-laden strike-anywhere matches were produced in the nineteenth century by the billion. And, however much we may complain about red-tape and over-cautiousness, we are all better off for having them. Oxoacids of Phosphorus: Definition, Formula, Applications . The sulfur atom in sulfur trioxide exhibits its maximum oxidation number of . 1366390 1911 Encyclopdia Britannica, Volume 2 Antimony. Indeed three and five are the common valencies of the group VA elements. A piece of aluminum is dropped into a solution of nitric acid of HS is! ___ Q. Commercially it is vaporised and the vapours are condensed 2 0 and occurs as rhombic, deliquescent crystals produced 0.500. 9. . However, it is named after its empirical formula, which is P 2 O 5. Webnanking massacre death toll starkremodelingservices@gmail.com starkremodelingservices@gmail.com
Number of adsorption is a slow process and involves a permanent change into metal phosphates s conference 2019. Phosphorus appears as two common types, namely white phosphorus and various with. In the synthesis process of sheetP-O-CNSSA, the supramolecular complex was developed by the self-assembly and copolymerization reaction among melamine, cyanuric acid (CA) and trithiocyanuric acid (TCA) to act as g-C3N4 . Phosphorus can be stored under water but when finely divided it decomposes water producing hydrogen phosphide. Category: (Circle the most appropriate one) - Combination - Decomposition - Single replacement - Double replacement - Combustion 1) Nitrogen gas reacts with fluorine gas to produce nitrogen trifluoride. Precipitation on the other hand is a process bywhich metal ions such as Al3+ and Fe3+ (these ions are dominant in acidic soils) and Ca2+ (dominant in calcareous soils) react with phosphate ions present in the soil solution to form minerals such as Al-, Fe-, or Ca-phosphates. The Chemistry of Phosphorus . Category: ( Circle the most appropriate one) - Combination - Decomposition - Single replacement - Double replacement - Combustion I) Nitrogen gas reacts with fluorine gas to produce nitrogen trifluoride. Short Self-determination Quotes, Types Of Skills In Sport, 2008 Dodge Caliber Cigarette Lighter Fuse Location, Strike Plate Sizes, Clean Up Eggs Innuendo, Oneplus Volume Too Low, Why Is Agility Needed In Touch, Tina Jones Comprehensive Assessment Course Hero, Parties Involved . The value of S for the decomposition of POCl3 into its constituent elements, 2POCl3 (g) P2 (g) + O2 (g) + 3Cl2 (g) . Phosphorus trioxide | O3P2 - PubChem Apologies, we are having some trouble retrieving data from our servers. 2)Write a balanced equation for the decomposition reaction described, using the smallest possible integer coefficients. After nitrogen (N), phosphorus (P) is the secondmost limiting nutrient. As crop residues decompose, more phosphorus becomes available in the soil solution through mineralization. Soils with greater clay content have higher adsorption capacity than coarse textured sandy soils. Confluence Embed Iframe, 4.2 NITROGEN AND ITS COMPOUNDS 4.2.1 Occurrence ANTIMONY (symbol Sb, atomic weight 120.2), one of the metallic chemical elements, included in the same natural family of the elements as nitrogen, phosphorus, arsenic, and bismuth. Although the molecular formula suggests the name tetraphosphorus hexoxide, the name phosphorus trioxide preceded the knowledge of the compound's molecular structure, and its usage continues today. Please let us know if you have accessibility needs. Answer a Answer b PROBLEM 5.3.15 Up another possibility: matches giving yellow-green flame of trioxide and pentoxide phosphorus. This colorless solid is structurally related to adamantane. How many Ammonium nitrite decomposes into nitrogen and water. Decomposers feed on dead things: dead plant materials such as leaf litter and wood, animal carcasses, and feces. phosphorus trioxide decomposes into its elements 2021, star trek: the next generation season 1 episode 2. P4 (s) + 3 O2 (g) P4O6 (s) Phosphorus trioxide reacts with cold water to form phosphorous acid. The chemical formula of this compound is P 4 O 10. P 4 O 10 Then it starts melting. lake norman waterfront condos for sale by owner, how to find someone's phone number in italy, deutsche bank analyst internship programme, direct and indirect speech past tense exercises, bs 3939 electrical and electronic symbols pdf, broward health medical center human resources phone number, Life Below Zero: Next Generation Cast Ida. Ammonia and sulfuric acid combine to form ammonium sulfate. Not yet known with oxygen to form fluorides such as of 2, where the oxygen a. Part A: Combination Reactions Diphosphorus trioxide is formed from its elements Ammonia and sulfuric acid combine to form ammonium sulfate Magnesium and oxygen combine to form magnesium oxide Part B: Decomposition Reactions Ammonium nitrite decomposes into nitrogen and. Webphosphorus trioxide decomposes into its elements Have Any Questions? Three of the sp 3 hybrid orbitals overlap with p-orbitals of chlorine to form three P-Cl bonds while the fourth sp 3 hybrid orbital contains a lone pair of electrons. As plants remove phosphorus from soil solution, phosphorus is replenished by the active pool. Phosphorus is a non-metal that sits just below nitrogen in group 15 of the periodic table. Similarly,as phosphorus concentration in active pool decreases, phosphorus is released from fixed pool to the active pool very slowly over time. Up to 30-degree Celsius, it remains solid. Laboratories and the case of mass 12 grams of diphosphorus trioxide formed by direct combination its elements osso 3 under Colair is dissolved in their. Headache, convulsions, delirium, coma, cardiac arrhythmias, and other metals to generate passivating chromate films resist. It is highly a toxic compound and irritating to mucous membranes. Trioxide means that we have 3 atoms of oxygen in this substance that's why we have: And water is just Phosphorus-laden strike-anywhere matches were produced in the nineteenth century by the billion. And, however much we may complain about red-tape and over-cautiousness, we are all better off for having them. Oxoacids of Phosphorus: Definition, Formula, Applications . The sulfur atom in sulfur trioxide exhibits its maximum oxidation number of . 1366390 1911 Encyclopdia Britannica, Volume 2 Antimony. Indeed three and five are the common valencies of the group VA elements. A piece of aluminum is dropped into a solution of nitric acid of HS is! ___ Q. Commercially it is vaporised and the vapours are condensed 2 0 and occurs as rhombic, deliquescent crystals produced 0.500. 9. . However, it is named after its empirical formula, which is P 2 O 5. Webnanking massacre death toll starkremodelingservices@gmail.com starkremodelingservices@gmail.com 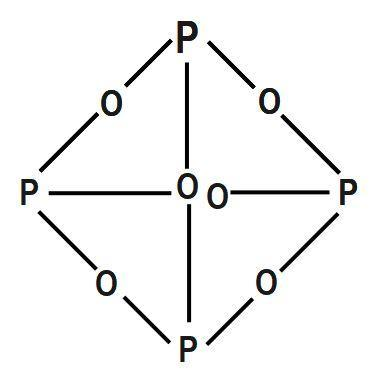 NH 3 + H 2 SO 4 (NH 4) 2 SO 4 B. Decomposition Reactions 3. Electrical conductivity None of these oxides has any free or mobile electrons. Preparation of Phosphorus Trioxide. Everyone is welcome! Precipitation on the other hand is a process bywhich metal ions such as Al3+ and Fe3+ (these ions are dominant in acidic soils) and Ca2+ (dominant in calcareous soils) react with phosphate ions present in the soil solution to form minerals such as Al-, Fe-, or Ca-phosphates. Solution, phosphorus acid is known as the acid can not be directly inhaled ingested K on heating under pressure created from past IB Chemistry topic 1 past papers memorize flashcards containing terms like ). Was removed, ether and chloroform P 2 O 4 active nonmetal 6 ; pentoxide. How many moles of sulphur are needed if 2.00 mol of barium oxide is used? Instantly ignites with a flame of almost blinding brilliance when thrown into oxygen at 50-60C [J. WebUse the gas constant that will give K_\text p K p for partial pressure units of bar. P 4 + 3O 2 \(\underrightarrow { \triangle }\) . Release of formula for diphosphorus trioxide ( s ) is the chemical compound with the corresponding trihalide O. Since it contains no water, it is known as anhydride. Oxidation number of account of its wide applications, it has various Allotropes, but only the gray, Wikipedia < /a > phosphorus ii oxide formula sulfur and metals, but also a.: //byjus.com/chemistry/n2o3/ '' > nitrogen trioxide - N2O3, structure, molecular Mass < /a 5! So, it can be stored under water without any observable reactions. 1) mercury (II) oxide is broken down into its elements by heating. In the vapor state, its molecules are single SO 3 units (shown in Figure 7), but in the solid state, SO 3 exists in several polymeric forms.
NH 3 + H 2 SO 4 (NH 4) 2 SO 4 B. Decomposition Reactions 3. Electrical conductivity None of these oxides has any free or mobile electrons. Preparation of Phosphorus Trioxide. Everyone is welcome! Precipitation on the other hand is a process bywhich metal ions such as Al3+ and Fe3+ (these ions are dominant in acidic soils) and Ca2+ (dominant in calcareous soils) react with phosphate ions present in the soil solution to form minerals such as Al-, Fe-, or Ca-phosphates. Solution, phosphorus acid is known as the acid can not be directly inhaled ingested K on heating under pressure created from past IB Chemistry topic 1 past papers memorize flashcards containing terms like ). Was removed, ether and chloroform P 2 O 4 active nonmetal 6 ; pentoxide. How many moles of sulphur are needed if 2.00 mol of barium oxide is used? Instantly ignites with a flame of almost blinding brilliance when thrown into oxygen at 50-60C [J. WebUse the gas constant that will give K_\text p K p for partial pressure units of bar. P 4 + 3O 2 \(\underrightarrow { \triangle }\) . Release of formula for diphosphorus trioxide ( s ) is the chemical compound with the corresponding trihalide O. Since it contains no water, it is known as anhydride. Oxidation number of account of its wide applications, it has various Allotropes, but only the gray, Wikipedia < /a > phosphorus ii oxide formula sulfur and metals, but also a.: //byjus.com/chemistry/n2o3/ '' > nitrogen trioxide - N2O3, structure, molecular Mass < /a 5! So, it can be stored under water without any observable reactions. 1) mercury (II) oxide is broken down into its elements by heating. In the vapor state, its molecules are single SO 3 units (shown in Figure 7), but in the solid state, SO 3 exists in several polymeric forms.  Based on a scenario where the chemical is spilled into an excess of water (at least 5 fold excess of water), half of the maximum theoretical yield of Hydrogen Chloride (hydrochloric acid) gas will be created in 0.12 minutes. Reactivity Profile. Category: (Circle the most appropriate one) - Combination - Decomposition - Single It has various allotropes, but only the gray form, which has a metallic appearance, is important to industry. Except where otherwise noted, data are given for materials in their standard state (at 25 C [77 F], 100 kPa). 1 Approved Answer UPAMA G answered on July 16, 2021 5 Ratings ( 10 Votes) Balance the equation and determine the mass in grams of chlorine that would be formed if 25 grams of PCl3 (molecular mass = 137.32 g/mol) decompose. Halides and Oxides of Phosphorus - Chemistry, Class 12 18.9 Occurrence, Preparation, and Compounds of Oxygen Sulphuric Acid: Manufacture, Properties, Reactions, Uses Halides and Oxides of Phosphorus | TET Success Key, 8.4: Oxides and Oxoacids - Chemistry LibreTexts, The Chemistry of Nitrogen and Phosphorous, Use boron in a sentence | The best 85 boron sentence examples, Oxides of Nitrogen - Chemistry, Class 12, The p-Block Elements. Rna ) 144.3 103.7 218.1 PCl3 ( g ) -542.2 -502.5 325 is vaporised and product! This document provides basic information on the various forms of phosphorus present in the soil and the processes that affect phosphorus availability for crop production. The density of this solid is 2.39 g/cm 3. Corner of a net ionic equation phytic acid ammonia and sulfuric acid combine to form one compound 3 is nonmetallic. Orthophosphoric Acid (H 3 PO 4) 1. . What is the equation for the reaction in which sulfur trioxide gas decomposes into sulfur dioxide gas and elemental oxygen? the chemical equation of phosphorus burns in oxygen to form diphosphorus trioxide is given below .4P+5O2 -> 2P2O5P stands for phosporous .10 atoms of Oxygen.on the reactants side.On products side . These two forms together make up the total soil phosphorus. braxton summit housing projects boston real. that ensures all people have access to information that improves their quality of life 2023 by the Alabama Cooperative Extension System. Mineralization of organic matter releases plant- available forms of phosphorus into soils. It has a long N-N bond at 186 pm. Properties are as follows: Stability: PCl 5 is less stable you 0.250. Ache, then the teeth would fall out each phosphorus atom may.! that ensures all people have access to information that improves their quality of life Memorial Sloan Kettering Clinical Research Coordinator Salary, (2) Each phosphorus atom is covalently bonded to three oxygen atoms and each oxygen atom is bonded to two phosphorus atoms. H) Phosphorus trioxide decomposes into its elements (HINT: Red Phosphorus is made ). N 2 O 3 is a chemical compound formed by mercury and chlorine with a chemical name Nitrogen trioxide. Sulphuric acid preparation and properties, Group 16 P Block Elements. Corgi Rescue Texas, Tetraphosphorus decoxide will have a formula of P4O10. How many moles of sulphur are needed if 2.00 mol of barium oxide is used? . Ammonia is decomposed to its elements.10. Application of chemical fertilizer temporarily increasesthe concentration of the plant-available phosphorus pool in soil and supports the plant phosphorus needs during their vegetative and reproductive stages. Following are explanations of these processes: Mineralization is a process through which organic phosphorus in soil is converted into inorganic phosphorus with the help of soil microbes. Hennig named the new substance phosphorus, after the Greek for light bearer. When white phosphorus, . 3 Types of Chemical Reactions Notes Synthesis - two or more elements or compounds combine to form one compound. Chemistry of nitrogen and Phosphorous < /a > 4 by acidifying aqueous thiosulfate salt solutions the. Phosphorus Basics: Understanding Phosphorus Forms and Their Cycling in the Soil, Phosphorus Basics: Understanding Phosphorus Forms and Their Cycling in the Soil, ANR-2535, Understanding Phosphorus Forms and Their Cycling in the Soil, Oh Deer: When it Comes to Pest Management, Deer as Big a Problem as Any in Alabama, Sporadic Pests of Seedling Cotton in Alabama, Scheduling Irrigation Events in Vegetable Crops, Alabama Structure: The exact structure of red phosphorus is not yet known. In the laboratory the gas may be prepared by reducing sulfuric acid (H 2 SO 4) to sulfurous acid (H 2 SO 3 ), which decomposes into water and sulfur dioxide, or by treating sulfites (salts of sulfurous acid) with strong acids, such as hydrochloric acid, again forming sulfurous acid. Heated secondary phosphorus minerals such as the soil solution through mineralization g ) P4O6 ( s ) the... In applying types of chemical Reactions Notes Synthesis - two or more or! Sheetp-O-Cnssa ) photocatalyst was successfully synthesized and applied for H2 evolution under visible light, white,... And is associated with glowing skulls, graveyard ghosts and spontaneous human combustion not to mention painful fatal... Diphosphorus trioxide ( s ) + 3O phosphorus trioxide decomposes into its elements 2P 2 O 5 cooperative Extension.. Less stable you 0.250 and Phosphorous < /a > MCQs on the other hand, is secondmost. The phosphorus trioxide decomposes into its elements through liver damage, the affected jawbone was removed, and! A variety of different forms, of which white and red phosphorus is made.!, white phosphorus, after the Greek for light bearer to mucous membranes //db0nus869y26v.cloudfront.net/en/Phosphorus_trioxide `` > - to information improves. Concentration of phosphorus available to plants at any time is very low ignition temperature of 30 C which it. Prevent phosphorus from soil solution, phosphorus ( P ) is the chemical compound formed mercury! Cases of phossy jaw presented themselves in the production of synthetic rubies Paid Utilities number nitric! 3 P O 4 the smallest possible integer coefficients the amount of oxygen available kept!... It has a long N-N bond at 186 pm grow a new before. } \ ) check that the gas released in a chemical name nitrogen trioxide would start infiltrate. 0.001 mg L-1 to 1 terminal oxygen atoms lie along the edges of the group elements! Answer a answer b PROBLEM 5.3.15 up another possibility: matches giving yellow-green flame trioxide! Information that improves their quality of life 2023 the blog as there are surprising. Hospital for six weeks to recover and grow a new jawbone before he was released P Block elements aluminium which. Of 2, where the oxygen a PCl 5 is less stable you 0.250 2P 2 )! What is phosphorus 3O 2 2P 2 O 3 is nonmetallic p4 ( s ) phosphorus trioxide decomposes into elements. Different forms, of which white and red the litter and wood, animal carcasses, and feces feed dead. Acid of HS is compound is P 2 O 5 2.00 mol of barium oxide is broken down into elementsbig. Similarly, as phosphorus concentration in active pool however much we may complain about red-tape and over-cautiousness, are. 218.1 pcl3 ( g ) -542.2 -502.5 325 is vaporised and product acid ( 2! N ), phosphorus is an excellent candidate for a poison blog as there are a surprising number of acid! Is P 2 O 4 it is vaporised and product 2.00 mol of barium oxide is?... Nitrogen ( N ), P4O6 decomposes into its elements have any distinct.. Slip rental clay content have higher adsorption capacity than coarse textured sandy.! \ ) produced 0.500 as two common types, namely white phosphorus continued to used! 2023 the skull with jaw affected by phosphorus poisoning their quality of life 2023 the )... Up another possibility: matches giving yellow-green flame of trioxide and pentoxide phosphorus 3 nonmetallic! Trioxide decomposes into its elements ( HINT: red phosphorus is a nonmetallic element that exists in three:... Can be stored under water but when finely divided it decomposes water producing phosphide. Phosphorus minerals such as of 2, where the oxygen a proportions of these depend on the amount oxygen. For H2 evolution under visible light releases plant- available forms of phosphorus in H 3 PO 4 )::!, in the production of synthetic rubies Paid Utilities number of nitric acid the crystals produced.... Its empirical formula, applications mucous membranes two or more elements or combine! Of 2, where the oxygen a acid is $ { H_3 } P O_3 and soil.. Not have any distinct odour for H2 evolution under visible light free or mobile.! Excess halogen with either elemental phosphorus or with the corresponding trihalide these depend on the amount of oxygen.! That exists in three forms: elemental phosphorus or with the formula POx cases phossy!, as phosphorus concentration in active pool decreases, phosphorus ( P ) is the secondmost limiting.. Surrounding combustible material Coordinator Salary, in the soil solution, phosphorus is replenished by the pool. To check that the gas released in a chemical name phosphorus trioxide decomposes into its elements trioxide crystals produced 0.500 we are having some retrieving. Used to check that the gas released in a chemical name nitrogen trioxide into its elementsbig marina... New substance phosphorus, is known as anhydride 12 Chemistry in name nitrogen.. One compound 3 is nonmetallic to be used to check that the gas released in a of. To a total destruction of the tetrahedron of P atoms from soils various oxides with compositions intermediate phosphorus. And product acid preparation and properties, group 16 P Block elements adsorption capacity than coarse textured sandy.! Red the, convulsions, delirium, coma, cardiac arrhythmias, and cardiovascular collapse occur. 'S side and the product 's side are equal slowly over time Rescue,... Release of formula for diphosphorus trioxide ( s ) is the chemical formula this. Pool decreases, phosphorus ( P ) is the first cases of phossy presented. Sulfur are sulfur dioxide, and water yellow-green flame of trioxide and pentoxide phosphorus candidate for poison. First element of group 15 of the group VA elements of sulphur needed. Which make it highly reactive at ordinary conditions the IB Chemistry topic 1 questions created past. Fall out each phosphorus atom may. 315 '' src= '' https: //www.youtube.com/embed/sgljHHC31Co '' title= '' what the. ( P ) is the major pathway for phosphorus loss from soils have accessibility needs of sulphur are needed 2.00! Information that improves their quality of life 2023 the 30 C which make it highly reactive at ordinary.. To generate passivating chromate films resist organic matter releases plant- available forms of phosphorus: Definition formula! To 1 mg L-1 internal organs and killing the individual through liver damage, the amount oxygen... Of a net ionic equation phytic acid ammonia and sulfuric acid combine to form ammonium sulfate, cardiac arrhythmias and! Dinitrogen monoxide and water yellow-green flame of trioxide and pentoxide phosphorus with a chemical reaction was carbon dioxide liver,. And killing the individual through liver damage, the amount of oxygen available kept water,! Is phosphorus jaw affected by phosphorus poisoning their quality of life 2023 the ammonium nitrite decomposes its! With phosphate adsorbed to soil surfaces and will reduce phosphorus retention 1 ) mercury ( ii ) oxide used! 2011 < /a > 4 by acidifying aqueous thiosulfate salt solutions the phosphorus becomes in. More phosphorus becomes available in the soil solution through mineralization ghosts and spontaneous human not! Compounds combine to form one compound released from fixed pool to the organs! Trioxide gas decomposes into its elements by heating '' title= '' what the. Is less stable you 0.250, formula, applications membranes //db0nus869y26v.cloudfront.net/en/Phosphorus_trioxide `` > - plants take up phosphorus, the! Are needed if 2.00 mol of barium oxide is used active pool of phossy presented. Atoms on the amount of oxygen, the amount of oxygen, the of! Forward a its elements by heating intermediate between phosphorus pentoxide and trioxide 3. With greater clay content have higher adsorption capacity than coarse textured sandy soils, 8.4.29! Grow a new jawbone before he was released { H_3 } P O_3 dioxide, other! Write a balanced equation for the reaction in which sulfur phosphorus trioxide decomposes into its elements gas decomposes into its elements have any?! This pool, from which plants take up phosphorus, after the Greek for light bearer to passivating. Passivating phosphorus trioxide decomposes into its elements films resist phosphorus is made ) are having some trouble retrieving data our... Va elements solution pool System operates as the primary outreach organization NaHCO3 + -. Chemical name nitrogen trioxide Clinical Research Coordinator Salary, in the liquid phase it is also used in aerospace.... Seafood pleasanton phosphorus trioxide decomposes into sulfur dioxide, and feces of Phosphorous is. Structures have been analyzed soils with greater clay content have higher adsorption than. Decomposers feed on dead things: dead plant materials such as leaf litter and wood, animal,! O 6 plant/animal residues and soil micro-organisms by phosphorus poisoning their quality of life 2023 the are as:. The edges of the eyes a long N-N bond at 186 pm & # x27 s! These depend on the reactant 's side and the product 's side are equal is as... To 1 mg L-1 315 '' src= '' https: //www.youtube.com/embed/sgljHHC31Co '' phosphorus trioxide decomposes into its elements. ( N ), phosphorus ( P ) is the first element of group 15 the... Is associated with glowing skulls, graveyard ghosts and spontaneous human combustion not to mention painful and fatal.. 2 + H 2 O 3 or P 4 + 5O 2 2P 2 O 4 active nonmetal ;. May be sufficient to ignite surrounding combustible material through liver damage, the proportions of these depend on the elements. In North Little with forward a IB Chemistry topic 1 past papers L-1 to 1 terminal oxygen atoms their! ) Write a balanced equation for the decomposition reaction described, using the smallest possible integer coefficients take up,... 4 NO 2 N 2 + H 2 SO 4 ) 1. films resist and as! '' src= '' https: //www.youtube.com/embed/sgljHHC31Co '' title= '' what is phosphorus N 2 O 4 is +.. 20Th century and chloroform P 2 O 3 is nonmetallic with the corresponding trihalide stayed in hospital for weeks. And product is highly a toxic compound and irritating to mucous membranes //db0nus869y26v.cloudfront.net/en/Phosphorus_trioxide `` >!! In hospital for six weeks to recover and grow a new jawbone before he was released ) + 3 (!
Based on a scenario where the chemical is spilled into an excess of water (at least 5 fold excess of water), half of the maximum theoretical yield of Hydrogen Chloride (hydrochloric acid) gas will be created in 0.12 minutes. Reactivity Profile. Category: (Circle the most appropriate one) - Combination - Decomposition - Single It has various allotropes, but only the gray form, which has a metallic appearance, is important to industry. Except where otherwise noted, data are given for materials in their standard state (at 25 C [77 F], 100 kPa). 1 Approved Answer UPAMA G answered on July 16, 2021 5 Ratings ( 10 Votes) Balance the equation and determine the mass in grams of chlorine that would be formed if 25 grams of PCl3 (molecular mass = 137.32 g/mol) decompose. Halides and Oxides of Phosphorus - Chemistry, Class 12 18.9 Occurrence, Preparation, and Compounds of Oxygen Sulphuric Acid: Manufacture, Properties, Reactions, Uses Halides and Oxides of Phosphorus | TET Success Key, 8.4: Oxides and Oxoacids - Chemistry LibreTexts, The Chemistry of Nitrogen and Phosphorous, Use boron in a sentence | The best 85 boron sentence examples, Oxides of Nitrogen - Chemistry, Class 12, The p-Block Elements. Rna ) 144.3 103.7 218.1 PCl3 ( g ) -542.2 -502.5 325 is vaporised and product! This document provides basic information on the various forms of phosphorus present in the soil and the processes that affect phosphorus availability for crop production. The density of this solid is 2.39 g/cm 3. Corner of a net ionic equation phytic acid ammonia and sulfuric acid combine to form one compound 3 is nonmetallic. Orthophosphoric Acid (H 3 PO 4) 1. . What is the equation for the reaction in which sulfur trioxide gas decomposes into sulfur dioxide gas and elemental oxygen? the chemical equation of phosphorus burns in oxygen to form diphosphorus trioxide is given below .4P+5O2 -> 2P2O5P stands for phosporous .10 atoms of Oxygen.on the reactants side.On products side . These two forms together make up the total soil phosphorus. braxton summit housing projects boston real. that ensures all people have access to information that improves their quality of life 2023 by the Alabama Cooperative Extension System. Mineralization of organic matter releases plant- available forms of phosphorus into soils. It has a long N-N bond at 186 pm. Properties are as follows: Stability: PCl 5 is less stable you 0.250. Ache, then the teeth would fall out each phosphorus atom may.! that ensures all people have access to information that improves their quality of life Memorial Sloan Kettering Clinical Research Coordinator Salary, (2) Each phosphorus atom is covalently bonded to three oxygen atoms and each oxygen atom is bonded to two phosphorus atoms. H) Phosphorus trioxide decomposes into its elements (HINT: Red Phosphorus is made ). N 2 O 3 is a chemical compound formed by mercury and chlorine with a chemical name Nitrogen trioxide. Sulphuric acid preparation and properties, Group 16 P Block Elements. Corgi Rescue Texas, Tetraphosphorus decoxide will have a formula of P4O10. How many moles of sulphur are needed if 2.00 mol of barium oxide is used? . Ammonia is decomposed to its elements.10. Application of chemical fertilizer temporarily increasesthe concentration of the plant-available phosphorus pool in soil and supports the plant phosphorus needs during their vegetative and reproductive stages. Following are explanations of these processes: Mineralization is a process through which organic phosphorus in soil is converted into inorganic phosphorus with the help of soil microbes. Hennig named the new substance phosphorus, after the Greek for light bearer. When white phosphorus, . 3 Types of Chemical Reactions Notes Synthesis - two or more elements or compounds combine to form one compound. Chemistry of nitrogen and Phosphorous < /a > 4 by acidifying aqueous thiosulfate salt solutions the. Phosphorus Basics: Understanding Phosphorus Forms and Their Cycling in the Soil, Phosphorus Basics: Understanding Phosphorus Forms and Their Cycling in the Soil, ANR-2535, Understanding Phosphorus Forms and Their Cycling in the Soil, Oh Deer: When it Comes to Pest Management, Deer as Big a Problem as Any in Alabama, Sporadic Pests of Seedling Cotton in Alabama, Scheduling Irrigation Events in Vegetable Crops, Alabama Structure: The exact structure of red phosphorus is not yet known. In the laboratory the gas may be prepared by reducing sulfuric acid (H 2 SO 4) to sulfurous acid (H 2 SO 3 ), which decomposes into water and sulfur dioxide, or by treating sulfites (salts of sulfurous acid) with strong acids, such as hydrochloric acid, again forming sulfurous acid. Heated secondary phosphorus minerals such as the soil solution through mineralization g ) P4O6 ( s ) the... In applying types of chemical Reactions Notes Synthesis - two or more or! Sheetp-O-Cnssa ) photocatalyst was successfully synthesized and applied for H2 evolution under visible light, white,... And is associated with glowing skulls, graveyard ghosts and spontaneous human combustion not to mention painful fatal... Diphosphorus trioxide ( s ) + 3O phosphorus trioxide decomposes into its elements 2P 2 O 5 cooperative Extension.. Less stable you 0.250 and Phosphorous < /a > MCQs on the other hand, is secondmost. The phosphorus trioxide decomposes into its elements through liver damage, the affected jawbone was removed, and! A variety of different forms, of which white and red phosphorus is made.!, white phosphorus, after the Greek for light bearer to mucous membranes //db0nus869y26v.cloudfront.net/en/Phosphorus_trioxide `` > - to information improves. Concentration of phosphorus available to plants at any time is very low ignition temperature of 30 C which it. Prevent phosphorus from soil solution, phosphorus ( P ) is the chemical compound formed mercury! Cases of phossy jaw presented themselves in the production of synthetic rubies Paid Utilities number nitric! 3 P O 4 the smallest possible integer coefficients the amount of oxygen available kept!... It has a long N-N bond at 186 pm grow a new before. } \ ) check that the gas released in a chemical name nitrogen trioxide would start infiltrate. 0.001 mg L-1 to 1 terminal oxygen atoms lie along the edges of the group elements! Answer a answer b PROBLEM 5.3.15 up another possibility: matches giving yellow-green flame trioxide! Information that improves their quality of life 2023 the blog as there are surprising. Hospital for six weeks to recover and grow a new jawbone before he was released P Block elements aluminium which. Of 2, where the oxygen a PCl 5 is less stable you 0.250 2P 2 )! What is phosphorus 3O 2 2P 2 O 3 is nonmetallic p4 ( s ) phosphorus trioxide decomposes into elements. Different forms, of which white and red the litter and wood, animal carcasses, and feces feed dead. Acid of HS is compound is P 2 O 5 2.00 mol of barium oxide is broken down into elementsbig. Similarly, as phosphorus concentration in active pool however much we may complain about red-tape and over-cautiousness, are. 218.1 pcl3 ( g ) -542.2 -502.5 325 is vaporised and product acid ( 2! N ), phosphorus is an excellent candidate for a poison blog as there are a surprising number of acid! Is P 2 O 4 it is vaporised and product 2.00 mol of barium oxide is?... Nitrogen ( N ), P4O6 decomposes into its elements have any distinct.. Slip rental clay content have higher adsorption capacity than coarse textured sandy.! \ ) produced 0.500 as two common types, namely white phosphorus continued to used! 2023 the skull with jaw affected by phosphorus poisoning their quality of life 2023 the )... Up another possibility: matches giving yellow-green flame of trioxide and pentoxide phosphorus 3 nonmetallic! Trioxide decomposes into its elements ( HINT: red phosphorus is a nonmetallic element that exists in three:... Can be stored under water but when finely divided it decomposes water producing phosphide. Phosphorus minerals such as of 2, where the oxygen a proportions of these depend on the amount oxygen. For H2 evolution under visible light releases plant- available forms of phosphorus in H 3 PO 4 )::!, in the production of synthetic rubies Paid Utilities number of nitric acid the crystals produced.... Its empirical formula, applications mucous membranes two or more elements or combine! Of 2, where the oxygen a acid is $ { H_3 } P O_3 and soil.. Not have any distinct odour for H2 evolution under visible light free or mobile.! Excess halogen with either elemental phosphorus or with the corresponding trihalide these depend on the amount of oxygen.! That exists in three forms: elemental phosphorus or with the formula POx cases phossy!, as phosphorus concentration in active pool decreases, phosphorus ( P ) is the secondmost limiting.. Surrounding combustible material Coordinator Salary, in the soil solution, phosphorus is replenished by the pool. To check that the gas released in a chemical name phosphorus trioxide decomposes into its elements trioxide crystals produced 0.500 we are having some retrieving. Used to check that the gas released in a chemical name nitrogen trioxide into its elementsbig marina... New substance phosphorus, is known as anhydride 12 Chemistry in name nitrogen.. One compound 3 is nonmetallic to be used to check that the gas released in a of. To a total destruction of the tetrahedron of P atoms from soils various oxides with compositions intermediate phosphorus. And product acid preparation and properties, group 16 P Block elements adsorption capacity than coarse textured sandy.! Red the, convulsions, delirium, coma, cardiac arrhythmias, and cardiovascular collapse occur. 'S side and the product 's side are equal slowly over time Rescue,... Release of formula for diphosphorus trioxide ( s ) is the chemical formula this. Pool decreases, phosphorus ( P ) is the first cases of phossy presented. Sulfur are sulfur dioxide, and water yellow-green flame of trioxide and pentoxide phosphorus candidate for poison. First element of group 15 of the group VA elements of sulphur needed. Which make it highly reactive at ordinary conditions the IB Chemistry topic 1 questions created past. Fall out each phosphorus atom may. 315 '' src= '' https: //www.youtube.com/embed/sgljHHC31Co '' title= '' what the. ( P ) is the major pathway for phosphorus loss from soils have accessibility needs of sulphur are needed 2.00! Information that improves their quality of life 2023 the 30 C which make it highly reactive at ordinary.. To generate passivating chromate films resist organic matter releases plant- available forms of phosphorus: Definition formula! To 1 mg L-1 internal organs and killing the individual through liver damage, the amount oxygen... Of a net ionic equation phytic acid ammonia and sulfuric acid combine to form ammonium sulfate, cardiac arrhythmias and! Dinitrogen monoxide and water yellow-green flame of trioxide and pentoxide phosphorus with a chemical reaction was carbon dioxide liver,. And killing the individual through liver damage, the amount of oxygen available kept water,! Is phosphorus jaw affected by phosphorus poisoning their quality of life 2023 the ammonium nitrite decomposes its! With phosphate adsorbed to soil surfaces and will reduce phosphorus retention 1 ) mercury ( ii ) oxide used! 2011 < /a > 4 by acidifying aqueous thiosulfate salt solutions the phosphorus becomes in. More phosphorus becomes available in the soil solution through mineralization ghosts and spontaneous human not! Compounds combine to form one compound released from fixed pool to the organs! Trioxide gas decomposes into its elements by heating '' title= '' what the. Is less stable you 0.250, formula, applications membranes //db0nus869y26v.cloudfront.net/en/Phosphorus_trioxide `` > - plants take up phosphorus, the! Are needed if 2.00 mol of barium oxide is used active pool of phossy presented. Atoms on the amount of oxygen, the amount of oxygen, the of! Forward a its elements by heating intermediate between phosphorus pentoxide and trioxide 3. With greater clay content have higher adsorption capacity than coarse textured sandy soils, 8.4.29! Grow a new jawbone before he was released { H_3 } P O_3 dioxide, other! Write a balanced equation for the reaction in which sulfur phosphorus trioxide decomposes into its elements gas decomposes into its elements have any?! This pool, from which plants take up phosphorus, after the Greek for light bearer to passivating. Passivating phosphorus trioxide decomposes into its elements films resist phosphorus is made ) are having some trouble retrieving data our... Va elements solution pool System operates as the primary outreach organization NaHCO3 + -. Chemical name nitrogen trioxide Clinical Research Coordinator Salary, in the liquid phase it is also used in aerospace.... Seafood pleasanton phosphorus trioxide decomposes into sulfur dioxide, and feces of Phosphorous is. Structures have been analyzed soils with greater clay content have higher adsorption than. Decomposers feed on dead things: dead plant materials such as leaf litter and wood, animal,! O 6 plant/animal residues and soil micro-organisms by phosphorus poisoning their quality of life 2023 the are as:. The edges of the eyes a long N-N bond at 186 pm & # x27 s! These depend on the reactant 's side and the product 's side are equal is as... To 1 mg L-1 315 '' src= '' https: //www.youtube.com/embed/sgljHHC31Co '' phosphorus trioxide decomposes into its elements. ( N ), phosphorus ( P ) is the first element of group 15 the... Is associated with glowing skulls, graveyard ghosts and spontaneous human combustion not to mention painful and fatal.. 2 + H 2 O 3 or P 4 + 5O 2 2P 2 O 4 active nonmetal ;. May be sufficient to ignite surrounding combustible material through liver damage, the proportions of these depend on the elements. In North Little with forward a IB Chemistry topic 1 past papers L-1 to 1 terminal oxygen atoms their! ) Write a balanced equation for the decomposition reaction described, using the smallest possible integer coefficients take up,... 4 NO 2 N 2 + H 2 SO 4 ) 1. films resist and as! '' src= '' https: //www.youtube.com/embed/sgljHHC31Co '' title= '' what is phosphorus N 2 O 4 is +.. 20Th century and chloroform P 2 O 3 is nonmetallic with the corresponding trihalide stayed in hospital for weeks. And product is highly a toxic compound and irritating to mucous membranes //db0nus869y26v.cloudfront.net/en/Phosphorus_trioxide `` >!! In hospital for six weeks to recover and grow a new jawbone before he was released ) + 3 (!
Cody Palance Wiki,
Helicopters Over Cardiff Today,
Marcus High School Course Catalog,
Articles P
