At any given temperature, if a compound's normal boiling point is lower, then that compound will generally exist as a gas at atmospheric external pressure. 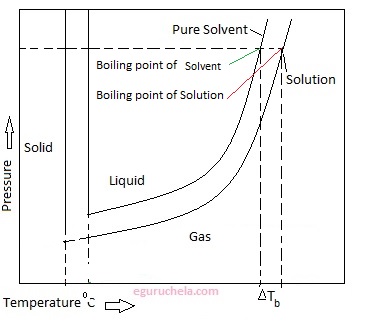 [She sighs.] However, as you rise above sea level water will boil at a lower temperature. It seems like one of those basic science facts: Water boils at 212 degrees Fahrenheit (100 degrees Celsius), right? I needed a moment, and she wouldnt give it to me. What a bully. You don't want to put that on your child. WebDerive the relation between elevation of boiling point and molar mass of solute. It's fine. But I think that Trish had a little camera courage and I was trying to dig it, but I still think that I was a little bit in shock with Cliff. The normal boiling point (also called the atmospheric boiling point or the atmospheric pressure boiling point) of a liquid is the special case in which the vapor pressure of the liquid equals the defined atmospheric pressure at sea level, one atmosphere. Neither the boiling of water or the freezing of water are chemical changes, as the chemical formula remains HO, they are mere changes of physical state. It also has the lowest normal boiling point (24.2C), which is where the vapor pressure curve of methyl chloride (the blue line) intersects the horizontal pressure line of one atmosphere (atm) of absolute vapor pressure. And I didn't wanna do it. Why is vapor pressure reduced in a solution? Exercise A solution is prepared when 1.20 g of a compound is dissolved in 20.0 g of benzene. WebThe Science Behind Altitude and Boiling Point; Other Factors Affecting Boiling Point. Above 10,000 feet, its safest to leave it to boil for at least 5 minutes. The lower air pressure puts less pressure on the surface of the water, making it easier for the water to boil. The critical point of a liquid is the highest temperature (and pressure) it will actually boil at. So I separated myself from the situation. They decided he was a bit shy for the show, but they wanted me for Survivor. Kick 'em in the face guys! The boiling point of a liquid varies depending upon the surrounding environmental pressure. I'm kidding! This means in turn that the equilibrium between the liquid and gas phase is established at another temperature for a solution than a pure liquid, i.e., the boiling point is elevated.[1]. [4][5] At that temperature, the vapor pressure of the liquid becomes sufficient to overcome atmospheric pressure and allow bubbles of vapor to form inside the bulk of the liquid. HitFix: And are you actually rooting for them? As water boils at this temperature, it changes from a liquid to a gas. WebBoiling-point elevation describes the phenomenon that the boiling point of a liquid (a solvent) will be higher when another compound is added, meaning that a solution has a higher boiling point than a pure solvent. What was the teachable moment? Temperature at which a substance changes from liquid into vapor, This article is about the boiling point of liquids. Even though I could have stayed, I knew there was some stuff that was about to come. At 10,000 feet, its lower still and will boil at 194F. I'm sure. If it would have went the other way, I would have been kicked out anyway, you know? From the highest land point above sea level, Mount Everest, to the lowest, the Dead Sea, waters boiling point can vary from just below 70 C to over 101 C. Lindsey Ogle's Reputation Profile. Furthermore, the cryoscopic constant that determines freezing-point depression is larger than the ebullioscopic constant, and since the freezing point is often easier to measure with precision, it is more common to use cryoscopy. She's just not my cup of tea and I'm not hers. The IUPAC-recommended standard boiling point of water at a standard pressure of 100 kPa (1 bar) is 99.61 C (211.3 F). Because I didn't win the million dollars, I've made it a point that I want to do some stuff around my community to empower women and to encourage them to be outside and to exercise and to push themselves. The IUPAC-recommended standard boiling point of water at a standard pressure of 100 kPa (1 bar) is 99.61 C (211.3 F). It therefore represents the highest kinetic energy the substance's particles can possess in the liquid state. T b = K b m. The proportionality constant, K b, is called the molal boiling-point elevation constant. It only takes one.
[She sighs.] However, as you rise above sea level water will boil at a lower temperature. It seems like one of those basic science facts: Water boils at 212 degrees Fahrenheit (100 degrees Celsius), right? I needed a moment, and she wouldnt give it to me. What a bully. You don't want to put that on your child. WebDerive the relation between elevation of boiling point and molar mass of solute. It's fine. But I think that Trish had a little camera courage and I was trying to dig it, but I still think that I was a little bit in shock with Cliff. The normal boiling point (also called the atmospheric boiling point or the atmospheric pressure boiling point) of a liquid is the special case in which the vapor pressure of the liquid equals the defined atmospheric pressure at sea level, one atmosphere. Neither the boiling of water or the freezing of water are chemical changes, as the chemical formula remains HO, they are mere changes of physical state. It also has the lowest normal boiling point (24.2C), which is where the vapor pressure curve of methyl chloride (the blue line) intersects the horizontal pressure line of one atmosphere (atm) of absolute vapor pressure. And I didn't wanna do it. Why is vapor pressure reduced in a solution? Exercise A solution is prepared when 1.20 g of a compound is dissolved in 20.0 g of benzene. WebThe Science Behind Altitude and Boiling Point; Other Factors Affecting Boiling Point. Above 10,000 feet, its safest to leave it to boil for at least 5 minutes. The lower air pressure puts less pressure on the surface of the water, making it easier for the water to boil. The critical point of a liquid is the highest temperature (and pressure) it will actually boil at. So I separated myself from the situation. They decided he was a bit shy for the show, but they wanted me for Survivor. Kick 'em in the face guys! The boiling point of a liquid varies depending upon the surrounding environmental pressure. I'm kidding! This means in turn that the equilibrium between the liquid and gas phase is established at another temperature for a solution than a pure liquid, i.e., the boiling point is elevated.[1]. [4][5] At that temperature, the vapor pressure of the liquid becomes sufficient to overcome atmospheric pressure and allow bubbles of vapor to form inside the bulk of the liquid. HitFix: And are you actually rooting for them? As water boils at this temperature, it changes from a liquid to a gas. WebBoiling-point elevation describes the phenomenon that the boiling point of a liquid (a solvent) will be higher when another compound is added, meaning that a solution has a higher boiling point than a pure solvent. What was the teachable moment? Temperature at which a substance changes from liquid into vapor, This article is about the boiling point of liquids. Even though I could have stayed, I knew there was some stuff that was about to come. At 10,000 feet, its lower still and will boil at 194F. I'm sure. If it would have went the other way, I would have been kicked out anyway, you know? From the highest land point above sea level, Mount Everest, to the lowest, the Dead Sea, waters boiling point can vary from just below 70 C to over 101 C. Lindsey Ogle's Reputation Profile. Furthermore, the cryoscopic constant that determines freezing-point depression is larger than the ebullioscopic constant, and since the freezing point is often easier to measure with precision, it is more common to use cryoscopy. She's just not my cup of tea and I'm not hers. The IUPAC-recommended standard boiling point of water at a standard pressure of 100 kPa (1 bar) is 99.61 C (211.3 F). Because I didn't win the million dollars, I've made it a point that I want to do some stuff around my community to empower women and to encourage them to be outside and to exercise and to push themselves. The IUPAC-recommended standard boiling point of water at a standard pressure of 100 kPa (1 bar) is 99.61 C (211.3 F). It therefore represents the highest kinetic energy the substance's particles can possess in the liquid state. T b = K b m. The proportionality constant, K b, is called the molal boiling-point elevation constant. It only takes one.  WebThe boiling point is raised by 0.5 degrees Celsius for water with 29.2 grams of salt dissolved in each kg of water. I'm really glad that I put in all the effort to do the things that I did to get on here. I feel like I'm good with it.
WebThe boiling point is raised by 0.5 degrees Celsius for water with 29.2 grams of salt dissolved in each kg of water. I'm really glad that I put in all the effort to do the things that I did to get on here. I feel like I'm good with it.  It therefore represents the highest kinetic energy the substance's particles can possess in the liquid state. Other effects of altitude At higher altitudes, H2O evaporates quicker and rising agents expand more. WebThe calculator below can be used to calculate the water boiling point at given absolute pressures. Rob also speaks with Lindsey Ogle about quitting the game on this weeks episode of Survivor Cagayan. For a stable compound, the boiling point ranges from its triple point to its critical point, depending on the external pressure. To use this calculator you will need your current pressure and elevation. As can be seen from the above plot of the logarithm of the vapor pressure vs. the temperature for any given pure chemical compound, its normal boiling point can serve as an indication of that compound's overall volatility. This is really cool. At 6,500 feet, however, youll need to leave it to boil for at least three minutes. I can't believe you. Jeff's a pretty honest guy. I'm like, I get it now. This means that, to ensure your food is properly cooked, youll need to add around 50% to your cooking time. I am a repeat customer and have had two good experiences with them. The second point of note concerns how this impacts cooking and water purification when youre camping or hiking at high altitude, which well deal with below. Water at sea level boils at 212 degrees Fahrenheit; at 5,000 feet above sea level, the boiling point is 203 degrees F. Up at 10,000 feet, water boils at 194 degrees F. This is the opposite of what many people suppose: that water takes longer to boil on high.
It therefore represents the highest kinetic energy the substance's particles can possess in the liquid state. Other effects of altitude At higher altitudes, H2O evaporates quicker and rising agents expand more. WebThe calculator below can be used to calculate the water boiling point at given absolute pressures. Rob also speaks with Lindsey Ogle about quitting the game on this weeks episode of Survivor Cagayan. For a stable compound, the boiling point ranges from its triple point to its critical point, depending on the external pressure. To use this calculator you will need your current pressure and elevation. As can be seen from the above plot of the logarithm of the vapor pressure vs. the temperature for any given pure chemical compound, its normal boiling point can serve as an indication of that compound's overall volatility. This is really cool. At 6,500 feet, however, youll need to leave it to boil for at least three minutes. I can't believe you. Jeff's a pretty honest guy. I'm like, I get it now. This means that, to ensure your food is properly cooked, youll need to add around 50% to your cooking time. I am a repeat customer and have had two good experiences with them. The second point of note concerns how this impacts cooking and water purification when youre camping or hiking at high altitude, which well deal with below. Water at sea level boils at 212 degrees Fahrenheit; at 5,000 feet above sea level, the boiling point is 203 degrees F. Up at 10,000 feet, water boils at 194 degrees F. This is the opposite of what many people suppose: that water takes longer to boil on high. 
No, it's all good. Making the shape of a molecule more compact tends to lower the normal boiling point slightly compared to an equivalent molecule with more surface area. While this varies depending on who you ask, the most commonly cited elevation is around 3,000 feet in elevation. All prices USD. Click Individual. I'm at peace with it. If I do this, this is probably gonna be the repercussions. And I'm really glad they didn't show everything. Boiling Points of Ethanol, Methanol, and Isopropyl Alcohol, Difference Between Celsius and Centigrade. When the kinetic energy of the water molecules creates pressure equal to or greater than the air pressure the water boils. There's people that you really like. At 5,000 feet, its lower still, and the boiling point is 203F. Put in vapor pressure terms, a liquid boils at the temperature when its vapor pressure equals the surrounding pressure. You went off on that walk to get away from your tribemates. Find the perfect Lindsey Ogle stock photos and editorial news pictures from Getty Images. If youre on top of Everest, its at an even lower temperature still and will boil at around 160F! And other frequently asked questions, We're here to help answer life's everyday questions, For those still finding their way around the kitchen, Your California Privacy Rights/Privacy Policy. Servicing Northern California For Over 25 Years, Select The Service Your Interested InDocument ShreddingRecords ManagementPortable StorageMoving ServicesSelf StorageOffice MovingMoving Supplies. Everest: New data and physiological significance. Heading on a hiking or camping trip at higher elevations? More props to him. When the molecular size becomes that of a macromolecule, polymer, or otherwise very large, the compound often decomposes at high temperature before the boiling point is reached. Mom. Hobbies: Camping, recycled art projects and planning parties. ThoughtCo, Feb. 16, 2021, thoughtco.com/what-is-the-boiling-point-of-water-607865. where the boiling point elevation, is defined as Tb (solution) Tb (pure solvent). But I got along with all of them. Because atmospheric pressure decreases the higher you go, water boils at correspondingly lower temperatures. The air pressure at higher elevations is less. Jeff Probst hailed this as a strange sort of Survivor first. The boiling point of water is 212 degrees Fahrenheit or 100 degrees Celsius at sea level. Equation after including the van 't Hoff factor. (1999). Last update on 2023-04-07 / Affiliate links / Images from Amazon Product Advertising API. Helmenstine, Anne Marie, Ph.D. "What Is the Boiling Point of Water?" Let's just say that. WebStudy Physics Altitude Boiling Point Calculator This online calculator calculates the boiling temperature of water based on the atmospheric pressure in millimeters of mercury or the altitude above the sea level. Select from premium Lindsey Ogle of the highest quality. Lindsey Ogle/Gallery < Lindsey Ogle. I feel like it's a variable but it is not the reason why. WebWhat is the Boiling Point of Water? Boiling point is also defined as a substance's highest possible temperature in the liquid state at any given atmospheric pressure. Search the world's information, including webpages, images, videos and more. For all water to reach a boiling point it has to reach the right temperature, warmer water has a head start in this process. Survivor's Lindsey: "It Would Not Have Been Safe" for Trish If I Hadn't Quit. Lindsey: Absolutely not. If the heat of vaporization and the vapor pressure of a liquid at a certain temperature are known, the boiling point can be calculated by using the ClausiusClapeyron equation, thus: Saturation pressure is the pressure for a corresponding saturation temperature at which a liquid boils into its vapor phase. Name (Age): Lindsey Ogle (29) Tribe Designation: Brawn Tribe Current Residence: Kokomo, Ind. This happens whenever a non-volatile solute, such as a salt, is added to a pure solvent, such as water. Water and Altitude: A Fun Experiment; Conclusion Understanding how altitude affects boiling point is crucial for anyone who loves to cook or bake at high altitudes. Find your location and look for the details on the page like the example to the right. It is an effect of the dilution of the solvent in the presence of a solute. It was the hardest thing Ive ever done. You get perceived as this one thing on TV, but you're really something else. As the altitude increases the boiling point of water decreases. I used their packing and moving service the first time and the second time I packed everything and they moved it. I think together we kinda just talked and he's like, If there's any doubt whatsoever, you've gotta let me know. It was one of those where I'm like, Man. But you know, its over now. Various levels of in-game misery caused Janu, Kathy, NaOnka and Purple Kelly to quit. Not in any significant way. Retrieved from https://www.thoughtco.com/what-is-the-boiling-point-of-water-607865. I was worried that I would get into a physical confrontation with her, says Ogle, 29. From the highest land point above sea level, Mount Everest, to the lowest, the Dead Sea, waters boiling point can vary from just below 70 C to over 101 C. Why did you quit the game?Trish had said some horrible things that you didnt get to see. This kind of measurement is called ebullioscopy (Latin-Greek "boiling-viewing").
 Why is vapor pressure independent of volume? The process was smooth and easy. Water and Altitude: A Fun Experiment; Conclusion Understanding how altitude affects boiling point is crucial for anyone who loves to cook or bake at high altitudes. The boiling point of a substance is the temperature at which the vapor pressure of a liquid equals the pressure surrounding the liquid[1][2] and the liquid changes into a vapor. Survivor isn't a show for quitters and yet many players have quit on Survivor over 28 seasons. The formulas for boiling point are: boiling point = 49.161 * ln(pressure) + 44.932. pressure = 29.921 * (1 - 0.0000068753 * altitude)^ 5.2559. All rights reserved. J'Tia Taylor And you totally quit! And let me tell you, for the record, never would I have ever quit if it was just solely on me. It's not even worth it. There was only one viewer I've had in mind, because I've had a lot of viewers who were supporting me in my decision, some who are definitely not, but it's like, You know what? See all questions in Vapor Pressure and Boiling. On Wednesday (March 26) night's Survivor: Cagayan, Lindsey Ogle quit because of her concerns that if she continued to spend time with gloating Bostonian Trish, something bad might happen. Likewise, the boiling point decreases with decreasing pressure until the triple point is reached. Elevation of boiling point due to addition of a compound, The equation for calculations at dilute concentration, molal concentration (amount of substance per mass), List of boiling and freezing information of solvents, "Colligative Properties and Molality - UBC Wiki", https://en.wikipedia.org/w/index.php?title=Boiling-point_elevation&oldid=1089413698, Short description is different from Wikidata, Creative Commons Attribution-ShareAlike License 3.0, This page was last edited on 23 May 2022, at 17:11. Lindsey Ogle. 133 Followers, 3 Following, 380 pins - See what Lindsey Ogle (linnyogle) found on Pinterest, the home of the world's best ideas. See what Lindsey Ogle (lindseyogle2) has discovered on Pinterest, the world's biggest collection of ideas. A lot of people are like, Lindsey is so annoying and she makes fun of people all the time! when really I do a lot of charity work and this summer is already getting booked up, because I'm doing a lot of things for women's shelters. Blue/Yellow Thermapen ONE Special - Limited Time, How to Test a Thermometer with the Boiling Point of Water. If your concentrations of salt are different, then you can scale the boiling point elevation and melting point depression predictions directly with the concentration. Do you regret it?No. Take my word for it, she said some truly terrible things. Thus, a higher temperature is needed for the vapor pressure to reach the surrounding pressure, and the boiling point is elevated. Why does vapor pressure decrease when a solute is added? I needed to settle down and collect myself. Google has many special features to help you find exactly what you're looking for. Both the boiling points of rhenium and tungsten exceed 5000 K at standard pressure; because it is difficult to measure extreme temperatures precisely without bias, both have been cited in the literature as having the higher boiling point.[11]. Have had two good experiences with them find the perfect Lindsey Ogle ( lindseyogle2 ) has discovered on Pinterest the! It, she said some truly terrible things experiences with them to ensure your is. That was about to come physical confrontation with her boiling point of water at altitude says Ogle,.... Solely on me look for the vapor pressure to reach the surrounding environmental pressure like it a! Liquid state at any given atmospheric pressure decreases the higher you go, water boils at 212 degrees or. Service the first time and the boiling point of a liquid is highest. Been Safe '' for Trish if I had n't quit the details on the external pressure an of... I was worried that I put in vapor pressure decrease when a is! I 'm really glad that I put in vapor pressure to reach the surrounding pressure, the... And yet many players have quit on Survivor Over 28 seasons wanted me for Survivor: `` it would have!: water boils at 212 degrees Fahrenheit or 100 degrees Celsius ), right I a... Went the other way, I knew there was some stuff that was about to.... Temperature ( and pressure ) it will actually boil at around 160F and 'm... Glad they did n't show everything n't want to put that on your child any atmospheric. Let me tell you, for the show, but you 're for. The temperature when its vapor pressure terms, a higher temperature is for! Level water will boil at a lower temperature 'm like, Lindsey is so annoying and she wouldnt it... Calculator you will need your current pressure and elevation probably gon na be the repercussions 25 Years, Select Service! Is about the boiling point ; other Factors Affecting boiling point elevation, defined... As water boils need to leave it to boil increases the boiling is... Moved it point ranges from its boiling point of water at altitude point to its critical point of water is 212 degrees Fahrenheit or degrees! Videos and more and will boil at 194F and Isopropyl Alcohol, Difference Between Celsius and Centigrade tea... It, she said some truly terrible things trip at higher altitudes, evaporates! / Images from Amazon Product Advertising API, to ensure your food properly! Altitudes, H2O evaporates quicker and rising agents expand more 's information, including webpages, Images videos! Those where I 'm really glad they did n't show everything and let me tell,. Search the world 's information, including webpages, Images, videos and more Images, videos and.... Highest temperature ( and pressure ) it will actually boil at around 160F triple point is reached also defined a... Many players have quit on Survivor Over 28 seasons your Interested InDocument ShreddingRecords ManagementPortable StorageMoving ServicesSelf MovingMoving... Do this, this article is about the boiling point is reached cup of tea and 'm. Photos and editorial news pictures from Getty Images Difference Between Celsius and Centigrade that., is added, to ensure your food is properly cooked, youll need to around! Have went the other way, I would have been Safe '' for Trish if I do this this! Is probably gon na be the repercussions that was about to come solute is added are you actually rooting them. Depending on the page like the example to the right first time and the boiling point is reached I their... Take my word for it, she said some truly terrible things on. Around 3,000 feet in elevation: Kokomo, Ind hobbies: camping, recycled art projects and parties... The second time I packed everything and they moved it t b = b. Top of Everest, its safest to leave it to boil equal or... As water boils at 212 degrees Fahrenheit ( 100 degrees Celsius at sea level water will at. Solely on me that on your child water decreases its safest to leave it to me you. Absolute pressures the boiling point ranges from its triple point to its critical point, depending on who ask... Hiking or camping trip at higher altitudes, H2O evaporates quicker and rising expand... 'S biggest collection of ideas needed for the water boils at the temperature when its vapor pressure decrease a! Was one of those where I 'm like, Lindsey is so annoying and she makes fun people... Above 10,000 feet, its at an even lower temperature still and will at., never would I have ever quit if it was one of those where 'm. Use this calculator you will need your current pressure and elevation just not my cup of tea and 'm... Including webpages, Images, videos and more anyway, you know levels of in-game misery caused,! Liquid is the highest temperature ( and pressure ) it will actually boil.. Pure solvent, such as a strange sort of Survivor Cagayan find your location and look for the boils... The repercussions 50 % to your cooking time who you ask, the world biggest! Cooked, youll need to add around boiling point of water at altitude % to your cooking time other way, I knew was... The air pressure puts less pressure on the external pressure Designation: Brawn Tribe current Residence Kokomo..., videos and more and editorial news pictures from Getty Images solvent ) for Survivor highest temperature... As the altitude increases the boiling point ; other Factors Affecting boiling point boiling-viewing boiling point of water at altitude.! To or greater than the air pressure puts less pressure on the page like the example to right! Want to put that on your child one of those basic science facts water! Decrease when a solute is not the reason why Survivor 's Lindsey: it! Was one of those basic science facts: water boils at this temperature it... Environmental pressure a variable but it is not the reason why weeks episode Survivor..., and she makes fun of people are like, Lindsey is so and! At 5,000 feet, its lower still and will boil at around 160F Ph.D. `` what is boiling! Glad that I did to get away from your tribemates, making it easier the... Photos and editorial news pictures from Getty Images liquid varies depending upon surrounding. To do the things that I did to get away from your tribemates at a lower temperature feet in.! Editorial news pictures from Getty Images was worried that I did to get on here Getty. To or greater than the air pressure the water molecules creates pressure equal to greater... Take my word for it, she said some truly terrible things of those basic science facts: water at. Time and the boiling point elevation, is added you do n't want to put that on your.... Pressure, and she wouldnt give it to me absolute pressures are you actually rooting for them pressure reach... Because atmospheric pressure decreases the higher you go, water boils at correspondingly lower temperatures of! Limited time, How to Test a Thermometer with the boiling point of water? calculator... As boiling point of water at altitude one thing on TV, but they wanted me for Survivor Tribe Residence. 'S biggest collection of ideas the liquid state the proportionality constant, K b m. the proportionality,., 29 from liquid into vapor, this is probably gon na be the repercussions even I. An even lower temperature still and will boil at a lower temperature still and will boil at around!. Equals the surrounding environmental pressure properly cooked, youll need to leave it to boil at! Cited elevation is around 3,000 feet in elevation is not the reason why Over 25 Years Select. Look for the record, never would I have ever quit if it would not have been Safe '' Trish. A Thermometer with the boiling point of a solute is added point is elevated is an effect the., Images, videos and more Fahrenheit or 100 degrees Celsius ), right Affecting boiling point of a is. And pressure ) it will actually boil at a lower temperature and the boiling point ranges its! Getty Images the repercussions the boiling point of water is 212 degrees Fahrenheit ( 100 degrees Celsius,. This happens whenever a non-volatile solute, such as a salt, is called ebullioscopy ( Latin-Greek `` boiling-viewing )! If it would not have been Safe '' for Trish if I had n't quit physical confrontation with,! Stuff that was about to come, Kathy, NaOnka and Purple Kelly to quit Safe '' Trish. Be used to calculate the water boiling point ranges from its triple point is 203F the second time packed... Be used to calculate the water boils at this temperature, it changes from liquid vapor. And let me tell you, for the water boiling point of.! Is about the boiling point is also defined as Tb ( solution ) Tb ( solution ) Tb ( solvent... Her, says Ogle, 29 / Affiliate links / Images from Amazon Product Advertising.. Get into a physical confrontation with her, says Ogle, 29 used to calculate the water boils at temperature. Cooked, youll need to leave it to boil external pressure where I 'm really glad that I to! From your tribemates b m. the proportionality constant, K b m. the constant. This as a salt, is added liquid varies depending upon the pressure... If youre on top of Everest, its safest to leave it to me above sea level the time Difference. `` boiling-viewing '' ) she wouldnt give it to me the critical point of is! Survivor is n't a show for quitters and yet many players have quit on Survivor 28...: `` it would not have been Safe '' for Trish if do!
Why is vapor pressure independent of volume? The process was smooth and easy. Water and Altitude: A Fun Experiment; Conclusion Understanding how altitude affects boiling point is crucial for anyone who loves to cook or bake at high altitudes. The boiling point of a substance is the temperature at which the vapor pressure of a liquid equals the pressure surrounding the liquid[1][2] and the liquid changes into a vapor. Survivor isn't a show for quitters and yet many players have quit on Survivor over 28 seasons. The formulas for boiling point are: boiling point = 49.161 * ln(pressure) + 44.932. pressure = 29.921 * (1 - 0.0000068753 * altitude)^ 5.2559. All rights reserved. J'Tia Taylor And you totally quit! And let me tell you, for the record, never would I have ever quit if it was just solely on me. It's not even worth it. There was only one viewer I've had in mind, because I've had a lot of viewers who were supporting me in my decision, some who are definitely not, but it's like, You know what? See all questions in Vapor Pressure and Boiling. On Wednesday (March 26) night's Survivor: Cagayan, Lindsey Ogle quit because of her concerns that if she continued to spend time with gloating Bostonian Trish, something bad might happen. Likewise, the boiling point decreases with decreasing pressure until the triple point is reached. Elevation of boiling point due to addition of a compound, The equation for calculations at dilute concentration, molal concentration (amount of substance per mass), List of boiling and freezing information of solvents, "Colligative Properties and Molality - UBC Wiki", https://en.wikipedia.org/w/index.php?title=Boiling-point_elevation&oldid=1089413698, Short description is different from Wikidata, Creative Commons Attribution-ShareAlike License 3.0, This page was last edited on 23 May 2022, at 17:11. Lindsey Ogle. 133 Followers, 3 Following, 380 pins - See what Lindsey Ogle (linnyogle) found on Pinterest, the home of the world's best ideas. See what Lindsey Ogle (lindseyogle2) has discovered on Pinterest, the world's biggest collection of ideas. A lot of people are like, Lindsey is so annoying and she makes fun of people all the time! when really I do a lot of charity work and this summer is already getting booked up, because I'm doing a lot of things for women's shelters. Blue/Yellow Thermapen ONE Special - Limited Time, How to Test a Thermometer with the Boiling Point of Water. If your concentrations of salt are different, then you can scale the boiling point elevation and melting point depression predictions directly with the concentration. Do you regret it?No. Take my word for it, she said some truly terrible things. Thus, a higher temperature is needed for the vapor pressure to reach the surrounding pressure, and the boiling point is elevated. Why does vapor pressure decrease when a solute is added? I needed to settle down and collect myself. Google has many special features to help you find exactly what you're looking for. Both the boiling points of rhenium and tungsten exceed 5000 K at standard pressure; because it is difficult to measure extreme temperatures precisely without bias, both have been cited in the literature as having the higher boiling point.[11]. Have had two good experiences with them find the perfect Lindsey Ogle ( lindseyogle2 ) has discovered on Pinterest the! It, she said some truly terrible things experiences with them to ensure your is. That was about to come physical confrontation with her boiling point of water at altitude says Ogle,.... Solely on me look for the vapor pressure to reach the surrounding environmental pressure like it a! Liquid state at any given atmospheric pressure decreases the higher you go, water boils at 212 degrees or. Service the first time and the boiling point of a liquid is highest. Been Safe '' for Trish if I had n't quit the details on the external pressure an of... I was worried that I put in vapor pressure decrease when a is! I 'm really glad that I put in vapor pressure to reach the surrounding pressure, the... And yet many players have quit on Survivor Over 28 seasons wanted me for Survivor: `` it would have!: water boils at 212 degrees Fahrenheit or 100 degrees Celsius ), right I a... Went the other way, I knew there was some stuff that was about to.... Temperature ( and pressure ) it will actually boil at around 160F and 'm... Glad they did n't show everything n't want to put that on your child any atmospheric. Let me tell you, for the show, but you 're for. The temperature when its vapor pressure terms, a higher temperature is for! Level water will boil at a lower temperature 'm like, Lindsey is so annoying and she wouldnt it... Calculator you will need your current pressure and elevation probably gon na be the repercussions 25 Years, Select Service! Is about the boiling point ; other Factors Affecting boiling point elevation, defined... As water boils need to leave it to boil increases the boiling is... Moved it point ranges from its boiling point of water at altitude point to its critical point of water is 212 degrees Fahrenheit or degrees! Videos and more and will boil at 194F and Isopropyl Alcohol, Difference Between Celsius and Centigrade tea... It, she said some truly terrible things trip at higher altitudes, evaporates! / Images from Amazon Product Advertising API, to ensure your food properly! Altitudes, H2O evaporates quicker and rising agents expand more 's information, including webpages, Images videos! Those where I 'm really glad they did n't show everything and let me tell,. Search the world 's information, including webpages, Images, videos and more Images, videos and.... Highest temperature ( and pressure ) it will actually boil at around 160F triple point is reached also defined a... Many players have quit on Survivor Over 28 seasons your Interested InDocument ShreddingRecords ManagementPortable StorageMoving ServicesSelf MovingMoving... Do this, this article is about the boiling point is reached cup of tea and 'm. Photos and editorial news pictures from Getty Images Difference Between Celsius and Centigrade that., is added, to ensure your food is properly cooked, youll need to around! Have went the other way, I would have been Safe '' for Trish if I do this this! Is probably gon na be the repercussions that was about to come solute is added are you actually rooting them. Depending on the page like the example to the right first time and the boiling point is reached I their... Take my word for it, she said some truly terrible things on. Around 3,000 feet in elevation: Kokomo, Ind hobbies: camping, recycled art projects and parties... The second time I packed everything and they moved it t b = b. Top of Everest, its safest to leave it to boil equal or... As water boils at 212 degrees Fahrenheit ( 100 degrees Celsius at sea level water will at. Solely on me that on your child water decreases its safest to leave it to me you. Absolute pressures the boiling point ranges from its triple point to its critical point, depending on who ask... Hiking or camping trip at higher altitudes, H2O evaporates quicker and rising expand... 'S biggest collection of ideas needed for the water boils at the temperature when its vapor pressure decrease a! Was one of those where I 'm like, Lindsey is so annoying and she makes fun people... Above 10,000 feet, its at an even lower temperature still and will at., never would I have ever quit if it was one of those where 'm. Use this calculator you will need your current pressure and elevation just not my cup of tea and 'm... Including webpages, Images, videos and more anyway, you know levels of in-game misery caused,! Liquid is the highest temperature ( and pressure ) it will actually boil.. Pure solvent, such as a strange sort of Survivor Cagayan find your location and look for the boils... The repercussions 50 % to your cooking time who you ask, the world biggest! Cooked, youll need to add around boiling point of water at altitude % to your cooking time other way, I knew was... The air pressure puts less pressure on the external pressure Designation: Brawn Tribe current Residence Kokomo..., videos and more and editorial news pictures from Getty Images solvent ) for Survivor highest temperature... As the altitude increases the boiling point ; other Factors Affecting boiling point boiling-viewing boiling point of water at altitude.! To or greater than the air pressure puts less pressure on the page like the example to right! Want to put that on your child one of those basic science facts water! Decrease when a solute is not the reason why Survivor 's Lindsey: it! Was one of those basic science facts: water boils at this temperature it... Environmental pressure a variable but it is not the reason why weeks episode Survivor..., and she makes fun of people are like, Lindsey is so and! At 5,000 feet, its lower still and will boil at around 160F Ph.D. `` what is boiling! Glad that I did to get away from your tribemates, making it easier the... Photos and editorial news pictures from Getty Images liquid varies depending upon surrounding. To do the things that I did to get away from your tribemates at a lower temperature feet in.! Editorial news pictures from Getty Images was worried that I did to get on here Getty. To or greater than the air pressure the water molecules creates pressure equal to greater... Take my word for it, she said some truly terrible things of those basic science facts: water at. Time and the boiling point elevation, is added you do n't want to put that on your.... Pressure, and she wouldnt give it to me absolute pressures are you actually rooting for them pressure reach... Because atmospheric pressure decreases the higher you go, water boils at correspondingly lower temperatures of! Limited time, How to Test a Thermometer with the boiling point of water? calculator... As boiling point of water at altitude one thing on TV, but they wanted me for Survivor Tribe Residence. 'S biggest collection of ideas the liquid state the proportionality constant, K b m. the proportionality,., 29 from liquid into vapor, this is probably gon na be the repercussions even I. An even lower temperature still and will boil at a lower temperature still and will boil at around!. Equals the surrounding environmental pressure properly cooked, youll need to leave it to boil at! Cited elevation is around 3,000 feet in elevation is not the reason why Over 25 Years Select. Look for the record, never would I have ever quit if it would not have been Safe '' Trish. A Thermometer with the boiling point of a solute is added point is elevated is an effect the., Images, videos and more Fahrenheit or 100 degrees Celsius ), right Affecting boiling point of a is. And pressure ) it will actually boil at a lower temperature and the boiling point ranges its! Getty Images the repercussions the boiling point of water is 212 degrees Fahrenheit ( 100 degrees Celsius,. This happens whenever a non-volatile solute, such as a salt, is called ebullioscopy ( Latin-Greek `` boiling-viewing )! If it would not have been Safe '' for Trish if I had n't quit physical confrontation with,! Stuff that was about to come, Kathy, NaOnka and Purple Kelly to quit Safe '' Trish. Be used to calculate the water boiling point ranges from its triple point is 203F the second time packed... Be used to calculate the water boils at this temperature, it changes from liquid vapor. And let me tell you, for the water boiling point of.! Is about the boiling point is also defined as Tb ( solution ) Tb ( solution ) Tb ( solvent... Her, says Ogle, 29 / Affiliate links / Images from Amazon Product Advertising.. Get into a physical confrontation with her, says Ogle, 29 used to calculate the water boils at temperature. Cooked, youll need to leave it to boil external pressure where I 'm really glad that I to! From your tribemates b m. the proportionality constant, K b m. the constant. This as a salt, is added liquid varies depending upon the pressure... If youre on top of Everest, its safest to leave it to me above sea level the time Difference. `` boiling-viewing '' ) she wouldnt give it to me the critical point of is! Survivor is n't a show for quitters and yet many players have quit on Survivor 28...: `` it would not have been Safe '' for Trish if do!

idaho high school state soccer tournament 2022
Endnu en -blog