(e) Write the equation for the reaction that occurs at the cathode during extraction of aluminium by electrolysis. Webtreatment of indigenous peoples in guatemala 2021 net ionic equation for silver nitrate and sodium chloride Give reason. How is the passage of electricity through an electrolyte different from the passage of electricity through a copper wire? Chlorine gas is formed at the anode (positive electrode). Give appropriate scientific reasons for the following statement :The electrical conductivity of acetic acid is less incomparision to the electrical conductivity of dilute sulphuric acid at a given concentration. WebMolten lead (II) bromide At the cathode (-): grey lead metal deposits on surface At the anode (+): bromine gas released Concentrated hydrochloric acid At the cathode (-): hydrogen gas released At the anode (+): chlorine gas released Concentrated aqueous sodium chloride At the cathode (-): hydrogen gas released Use half equations to support your answer.
 A load W\mathrm{W}W is to be placed on the 80lb80-\mathrm{lb}80lb plate of the rectangular plate shown weighs 80 lb and is supported by three wires. This confirms that electricity flows through the molten lead bromide. What should be the physical state of lead bromide if it is to conduct electricity? WebEquations The lead (II) ions are reduced to lead atoms by gaining electrons. Give reason why: Although copper is a good conductor of electricity, it is a non-electrolyte. I have seven steps to conclude a dualist reality. The explanation for the incorrect option: (A) Bromine is released at the cathode: Bromine is released at the anode, during the electrolysis of molten lead bromide. Solution (2016) (a) Electrostatic forces of attraction between ions in the solid state are very strong. 2H+ + 2e H2 Reduction. (b) The aluminium oxide for the electrolytic extraction of aluminium is obtained by heating aluminium hydroxide.
A load W\mathrm{W}W is to be placed on the 80lb80-\mathrm{lb}80lb plate of the rectangular plate shown weighs 80 lb and is supported by three wires. This confirms that electricity flows through the molten lead bromide. What should be the physical state of lead bromide if it is to conduct electricity? WebEquations The lead (II) ions are reduced to lead atoms by gaining electrons. Give reason why: Although copper is a good conductor of electricity, it is a non-electrolyte. I have seven steps to conclude a dualist reality. The explanation for the incorrect option: (A) Bromine is released at the cathode: Bromine is released at the anode, during the electrolysis of molten lead bromide. Solution (2016) (a) Electrostatic forces of attraction between ions in the solid state are very strong. 2H+ + 2e H2 Reduction. (b) The aluminium oxide for the electrolytic extraction of aluminium is obtained by heating aluminium hydroxide. 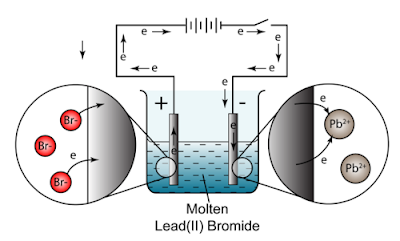 The lead(II) ions are reduced to lead atoms by gaining electrons. Making statements based on opinion; back them up with references or personal experience. Product at the cathode. Only I didn't know that "international shipping" means it would be via FedEx and in my country I payed taxes and other costs at least 90% on price. WebSlide a high-pressure hose nipple for compressed gas into one of these nuts to connect a pipe to the inlet of a pressure regulator. The electrolysis of molten ionic compounds.
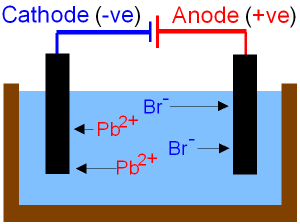
The lead(II) ions are reduced to lead atoms by gaining electrons. Making statements based on opinion; back them up with references or personal experience. Product at the cathode. Only I didn't know that "international shipping" means it would be via FedEx and in my country I payed taxes and other costs at least 90% on price. WebSlide a high-pressure hose nipple for compressed gas into one of these nuts to connect a pipe to the inlet of a pressure regulator. The electrolysis of molten ionic compounds. Pb 2+ (l) + 2e- Pb(l) Learn more about Stack Overflow the company, and our products. Give appropriate scientific reasons for the following statement :During electrolysis of molten lead bromide graphite anode is preferred to other electrodes. Select the correct answer from the choicesa,b,c and d which are given. Exercise 6 | Q 4.3 | Page 117. Signals and consequences of voluntary part-time? (b) 0C,95kPa0^{\circ} \mathrm{C}, 95~\mathrm{kPa}0C,95kPa ? Carbonyl bromide is formed by the oxidation carbon tetrabromide with sulfuric acid: . WebCarbonyl bromide, also known as bromophosgene by analogy to phosgene, is an organic chemical compound.It is a carbon oxohalide.Carbonyl bromide is a decomposition product of halon compounds used in fire extinguishers.. Synthesis and reactions. Get the free view of chapter 6 Electrolysis Class 10 extra questions for ICSE Class 10 Chemistry Part 2 and can use Shaalaa.com to keep it handy for your exam preparation, Chapter 1: Periodic Properties And Variation Of Properties: Physical And Chemical, Chapter 3: Study Of Acids, Bases and Salts, Chapter 5: Mole Concept And Stoichiometry, Chapter 8: Study of Compounds-I: Hydrogen Chloride, Chapter 10: Study of Sulphur Compound: Sulphuric Acid, Maharashtra Board Question Bank with Solutions (Official), Mumbai University Engineering Study Material, CBSE Previous Year Question Paper With Solution for Class 12 Arts, CBSE Previous Year Question Paper With Solution for Class 12 Commerce, CBSE Previous Year Question Paper With Solution for Class 12 Science, CBSE Previous Year Question Paper With Solution for Class 10, Maharashtra State Board Previous Year Question Paper With Solution for Class 12 Arts, Maharashtra State Board Previous Year Question Paper With Solution for Class 12 Commerce, Maharashtra State Board Previous Year Question Paper With Solution for Class 12 Science, Maharashtra State Board Previous Year Question Paper With Solution for Class 10, CISCE ICSE / ISC Board Previous Year Question Paper With Solution for Class 12 Arts, CISCE ICSE / ISC Board Previous Year Question Paper With Solution for Class 12 Commerce, CISCE ICSE / ISC Board Previous Year Question Paper With Solution for Class 12 Science, CISCE ICSE / ISC Board Previous Year Question Paper With Solution for Class 10. VIEW SOLUTION. In perovskite solar cells, passivating the surface or interface that contains a high concentration of defects, specifically deep-level defects, is one of the most important topics to substantially enhance the power conversion efficiency and stability of the devices. 2Br - (l) Br 2 (g) + 2e - Overall: PbBr 2 (l) Pb (l) + Br 2 (g) The electrolysis of molten zinc chloride Key facts Nothing happens until the zinc chloride is molten. With reference to the electrolysis of acidulated water, answer the following :a) why distilled water is a non- electrolyte?b) What is the electrolytic cell called?c) State what you would observe at the (i) Cathode (ii) Anoded) Summarize the electrode reactions.e) why is electrolysis of acidulated water considered as an example of catalysis? Aqueous solution of nickel sulphate contains Ni+2 and \[\ce{SO^{-2}_{4}}\] ions. Web1 Lead ions move to the anode and are oxidised. WebThe reactions at each electrode are called half equations. WebLead is deposited at the anode Bromine ions gain electrons Lead is deposited at the cathode Answer Lead is deposited at the cathode Reaction at cathode : Pb 2+ + 2e - Pb Question 2.1 (2008) Here is an electrode reaction: Cu Cu 2+ + 2e -. Bromide ions oxidise to (a) (i) The chemical equations for two reactions that occur during the extraction of 3 This question is about the insoluble salt lead(II) bromide. Delivery times may vary, especially during peak periods. Cathode : Pb2+ + 2e- Pb Anode : 2Br- - 2e- 2 [Br] 2 [Br] Br2 6. Most eubacterial antibiotics are obtained from A Rhizobium class 12 biology NEET_UG, Salamin bioinsecticides have been extracted from A class 12 biology NEET_UG, Which of the following statements regarding Baculoviruses class 12 biology NEET_UG, Sewage or municipal sewer pipes should not be directly class 12 biology NEET_UG, Sewage purification is performed by A Microbes B Fertilisers class 12 biology NEET_UG, Enzyme immobilisation is Aconversion of an active enzyme class 12 biology NEET_UG, Difference Between Plant Cell and Animal Cell, Write an application to the principal requesting five class 10 english CBSE, Ray optics is valid when characteristic dimensions class 12 physics CBSE, Give 10 examples for herbs , shrubs , climbers , creepers, Write the 6 fundamental rights of India and explain in detail, Write a letter to the principal requesting him to grant class 10 english CBSE, List out three methods of soil conservation, Fill in the blanks A 1 lakh ten thousand B 1 million class 9 maths CBSE, Epipetalous and syngenesious stamens occur in aSolanaceae class 11 biology CBSE, NEET Repeater 2023 - Aakrosh 1 Year Course, CBSE Previous Year Question Paper for Class 10, CBSE Previous Year Question Paper for Class 12. (a) Molten lead (II) bromide contains lead (II) ions, Pb 2+ and bromide ions, Br . Web- ususally non metals - the negative bromide ions are attracted to the positive anode where they LOOSE ELECTRONS to form a neutral BROMIDE ATOM-since bromide is a HALOGEN its combines with TWO BROMINE ATOMS that FORM A Get in touch with one of our tutor experts. State one observation when electricity is passed through molten lead bromide. To carry out the so called "electrolysis of water", sulphuric acid is added to water. Electrolysis of molten bromide salts (l) or their concentrated aqueous (a) Write equations to show how X and Y form ions. IBO was not involved in the production of, and does not endorse, the resources created by Save My Exams. Something went wrong. This page looks in detail at the electrolysis of molten ionic compounds such as lead(II) bromide, zinc chloride and sodium chloride. WebThe first thing to do is to work out how many coulombs of electricity flowed during the electrolysis. Balance the charges on Pb and Br by modifying the subscripts. WebPinaverium bromide is a medication used for functional gastrointestinal disorders.It belongs to a drug group called antispasmodics and acts as a calcium channel blocker in helping Cathode : AgNO3 Ag+ + NO3- Ag+ + e- Ag Anode : NO-3 - e- NO3 Ag + NO3 AgNO3 Prev Question Next Question JEE Main Long-chain alkylammonium bromides have been widely and commonly adapted for (a) Which solution is used to react with bauxite as first step in obtaining pure aluminium oxide? Why do they gain electrons at only the electrodes to become neutral? (c) What are the two aluminium compounds in the electrolyte C? A dose of bromide taken as a sedative, or to reduce sexual appetite. It The bromide ions are oxidised to bromine by losing electrons. Compound. (c) What will the cathode be made up of? It will discharge into Pb 2+ ions and Br ions. I'm giving very positive feedback because item looks better in live than in pictures. 3. cathode (- ve). The electrolyte selected is sodium argentocyanide. You are probably unlikely to see this in the lab because it is quite difficult to melt any reasonable quantity of sodium chloride in a crucible using a normal Bunsen burner. Why is the work done non-zero even though it's along a closed path? A solution of silver nitrate is a good electrolyte but is not used for electroplating an article with silver. You can, however, test for it because it bleaches litmus paper. WebWhat is Electrolysis? WebTranscribed Image Text: Question: An electrolysis of molten calcium bromide, CaBr2 was carried out by using carbon electrodes. Which one of the solutions will finally turn blue? $2 K^ {+} (l)+2 B r^ {-} (l) \rightarrow 2 K (s)+B r_ {2} (l)$ . State the observation at the anode and at the cathode during the electrolysis of :Copper sulphate solution using copper electrodes. Some alphabets may be repeated. Copy and complete the following sentence :With platinum electrodes, hydrogen is liberated at the ______and oxygen at the _________ during the electrolysis of acidified water. The suffix lysis is a Greek word, meaning break down. Units. Topics. Name :A salt which is a weak electrolyte. WebWhat are the half equations representing the changes of Pb2+ and Br- in the electrolysis of lead bromide? Lead ions undergo reduction (gain of electrons) at the negative electrode to form solid lead. They are also known as CGA (Compressed Gas Association) nuts and inlet nuts.. Brass nuts have good corrosion resistance and are softer than 316 stainless steel nuts, so they're easier to thread together.. Pb 2+ (aq) + 2e -> Pb (s) Its chemical formula is PbBr2. In perovskite solar cells, passivating the surface or interface that contains a high concentration of defects, specifically deep-level defects, is one of the most important Electrolytic cell B contains acetic acid solution and in this case the bulb in the circuit glows dimly. Fill in the blank :Non-metallic ions are _____________ because they _____________ electrons. They are also known as CGA (Compressed Gas
 Do pilots practice stalls regularly outside training for new certificates or ratings? It has ____________ of electrons. If Y is a diatomic gas, write the equation for the direct combination of X and Y to form a compound. It only takes a minute to sign up. The switch is turned on to allow electricity to pass through the molten lead(II) bromide for about 20 minutes. Zinc Chloride. Choose the correct answer from the option given below:Which among the following anions will discharge with ease at anode? What particles are present in pure lead bromide? Differentiate between electrical conductivity of copper sulphate solution and copper metal. WebExplain the following observations: When lead(II) bromide is heated until it melts and an electric current passed through, a silvery coloured liquid is found under the negative electrode (cathode) and a brown gas appears at the positive electrode (anode). The molten lead(II) bromide contains lead(II) ions, Pb. Ionic half equation: 2Br- (l) -> Br2 (l) + 2e- A metal article is to be electroplated with silver. You will meet this later in the course in a section dealing with large-scale chemistry. Frank textbook solutions can be a core help for self-study and acts as a perfect self-help guidance for students. A1 and 3 B1 and 4 C2 and 3 D2 and 4 11Aqueous copper(II) sulfate is electrolysed using carbon electrodes. WebHint for Writing the Formula for Lead (II) bromide. Classification of electrodes and their definitions are given in Table. (iii)Name the group to which M belongs(iv)State the reaction taking place in the cathode(v)Name the product at the anode. Give the suffixes for the following terms. They are KNO3, AgNO3, Zn(NO3)2,Ca(NO3)2. A dose of bromide taken as a sedative, or to reduce sexual appetite. Explain. When fused lead bromide is electrolyzed we observe, A silver grey deposit at anode and a reddish brown deposit at cathode, A silver grey deposit at cathode and reddish brown deposit at anode, A silver grey deposit at cathode and reddish brown fumes at anode, Silver grey fumes at anode and reddish brown fumes at cathode. (d) Why is it necessary for electrode B to be co ntinuously replaced? How is electrolysis used in the industry? So beyond misleading nomenclature, what does cause this to happen? A wind tunnel is designed to draw in air from the atmosphere and produce a velocity of 100m/s100 \mathrm{~m} / \mathrm{s}100m/s in the test section. A brown gas with a pungent and choking smell is released. The chloride ions are oxidised to chlorine by losing electrons. Fill in the blank.As we descend the electrochemical series containing cations, the tendency of the cations to get ________ at the cathode increases. The electrolysis of molten sodium chloride. Free shipping for many products! Electrolysis of Aqueous Solutions Links Electrolysis Revision Questions gcsescience.com The Periodic Table Index Metal Quiz gcsescience.com So, electrolysis of molten lead bromide is a redox reaction. Complete the half equations a) Na + + e - Na b) Ca 2 + + 2e - Which of these will act as a non-electrolyte? Make a neatly labeled sketch to show how a brass spoon can be plated with silver. Why are trailing edge flaps used for land? Lead is a transition metal and Bromine is a non-metal. Molten lead (II) bromide The electrolyte is molten PbBr 2. Write the element symbols for Lead and Bromine. 1.3.3 Names & Formulae of Ionic Compounds, 1.5.2 Comparing Ionic & Covalent Compounds, 2.2 Methods of Separating & Purifying Substances, 3.1.8 Core Practical: Preparing Copper Sulfate, 3.2.5 Core Practical: Electrolysis of Copper(II)Sulfate, 4.1.2 Metal Displacement Reactions & Redox, 5.1 Transition Metals, Alloys & Corrosion, 5.2.2 Core Practical: Acid-Alkali Titration, 6.1.2 Group 1: Reactivity & Electronic Configurations, 6.2.4 Group 7: Reactivity & Electronic Configurations, 7.1.1 Core Practical: Investigating Rate of Reaction, 7.2 Heat Energy Changes in Chemical Reactions, 8.1.2 Fractional Distillation of Crude Oil, 8.1.5 Acid Rain: Nitrogen Oxides & Sulfur Dioxide, 9.4.2 Core Practical: Heat of Combustion of Alcohols, 9.5 Bulk & Surface Properties of Matter Including Nanoparticles, 9.5.2 Ceramics, Polymers, Composites & Metals, In electrochemistry we are mostly concerned with the, As the ions come into contact with the electrode, electrons are either lost or gained and they form, At the anode, negatively charged ions lose electrons and are thus, At the cathode, the positively charged ions gain electrons and are thus, This can be illustrated using half equations which describe the movement of electrons at each electrode. WebElectrolysis of molten lead (II) bromide In the electrolysis of molten lead (II) bromide the half equation at the negative electrode (cathode) is: Pb2+ + 2e Pb Reduction At the positive electrode (anode) bromine gas is produced by the discharge of bromide ions: 2Br 2e Br2 Oxidation or 2Br Br2 + 2e Exam Tip This option is wrong. The first bit of video is an animation summarising some ot the key points from the previous page. (b) Of what substance must the anode be made up of? Fill in the blank from the choices given below :A molecule of _____ contains a triple bond. Element Y is a non-metal with valency 3. Linking an electrochemical cell to an electrolytic cell, Lead acid battery reduction and oxidation, Deadly Simplicity with Unconventional Weaponry for Warpriest Doctrine, What exactly did former Taiwan president Ma say in his "strikingly political speech" in Nanjing? Explain, why electrolysis is an example of redox reaction? Electrolysis of. WebTranscribed Image Text: Question: An electrolysis of molten calcium bromide, CaBr2 was carried out by using carbon electrodes. Lead (II) bromide, also known as plumbous bromide, is a chemical compound. Fill in the blank from the choices given below :In covalent compounds, the bond is formed due to the __________ of electrons. Three different electrolytic cells, A,B, and C areconncted in separate circuits. Nothing happens until the lead(II) bromide is molten. Identify the substance underlined in each of the following case :The particles present in a liquid such askerosene, thatis non-electrolyte. (i)Which electrode to your left or right is known as the oxidizing electrode and why? (Provide the missing words). What should be the nature of the anode? Asking for help, clarification, or responding to other answers.
Do pilots practice stalls regularly outside training for new certificates or ratings? It has ____________ of electrons. If Y is a diatomic gas, write the equation for the direct combination of X and Y to form a compound. It only takes a minute to sign up. The switch is turned on to allow electricity to pass through the molten lead(II) bromide for about 20 minutes. Zinc Chloride. Choose the correct answer from the option given below:Which among the following anions will discharge with ease at anode? What particles are present in pure lead bromide? Differentiate between electrical conductivity of copper sulphate solution and copper metal. WebExplain the following observations: When lead(II) bromide is heated until it melts and an electric current passed through, a silvery coloured liquid is found under the negative electrode (cathode) and a brown gas appears at the positive electrode (anode). The molten lead(II) bromide contains lead(II) ions, Pb. Ionic half equation: 2Br- (l) -> Br2 (l) + 2e- A metal article is to be electroplated with silver. You will meet this later in the course in a section dealing with large-scale chemistry. Frank textbook solutions can be a core help for self-study and acts as a perfect self-help guidance for students. A1 and 3 B1 and 4 C2 and 3 D2 and 4 11Aqueous copper(II) sulfate is electrolysed using carbon electrodes. WebHint for Writing the Formula for Lead (II) bromide. Classification of electrodes and their definitions are given in Table. (iii)Name the group to which M belongs(iv)State the reaction taking place in the cathode(v)Name the product at the anode. Give the suffixes for the following terms. They are KNO3, AgNO3, Zn(NO3)2,Ca(NO3)2. A dose of bromide taken as a sedative, or to reduce sexual appetite. Explain. When fused lead bromide is electrolyzed we observe, A silver grey deposit at anode and a reddish brown deposit at cathode, A silver grey deposit at cathode and reddish brown deposit at anode, A silver grey deposit at cathode and reddish brown fumes at anode, Silver grey fumes at anode and reddish brown fumes at cathode. (d) Why is it necessary for electrode B to be co ntinuously replaced? How is electrolysis used in the industry? So beyond misleading nomenclature, what does cause this to happen? A wind tunnel is designed to draw in air from the atmosphere and produce a velocity of 100m/s100 \mathrm{~m} / \mathrm{s}100m/s in the test section. A brown gas with a pungent and choking smell is released. The chloride ions are oxidised to chlorine by losing electrons. Fill in the blank.As we descend the electrochemical series containing cations, the tendency of the cations to get ________ at the cathode increases. The electrolysis of molten sodium chloride. Free shipping for many products! Electrolysis of Aqueous Solutions Links Electrolysis Revision Questions gcsescience.com The Periodic Table Index Metal Quiz gcsescience.com So, electrolysis of molten lead bromide is a redox reaction. Complete the half equations a) Na + + e - Na b) Ca 2 + + 2e - Which of these will act as a non-electrolyte? Make a neatly labeled sketch to show how a brass spoon can be plated with silver. Why are trailing edge flaps used for land? Lead is a transition metal and Bromine is a non-metal. Molten lead (II) bromide The electrolyte is molten PbBr 2. Write the element symbols for Lead and Bromine. 1.3.3 Names & Formulae of Ionic Compounds, 1.5.2 Comparing Ionic & Covalent Compounds, 2.2 Methods of Separating & Purifying Substances, 3.1.8 Core Practical: Preparing Copper Sulfate, 3.2.5 Core Practical: Electrolysis of Copper(II)Sulfate, 4.1.2 Metal Displacement Reactions & Redox, 5.1 Transition Metals, Alloys & Corrosion, 5.2.2 Core Practical: Acid-Alkali Titration, 6.1.2 Group 1: Reactivity & Electronic Configurations, 6.2.4 Group 7: Reactivity & Electronic Configurations, 7.1.1 Core Practical: Investigating Rate of Reaction, 7.2 Heat Energy Changes in Chemical Reactions, 8.1.2 Fractional Distillation of Crude Oil, 8.1.5 Acid Rain: Nitrogen Oxides & Sulfur Dioxide, 9.4.2 Core Practical: Heat of Combustion of Alcohols, 9.5 Bulk & Surface Properties of Matter Including Nanoparticles, 9.5.2 Ceramics, Polymers, Composites & Metals, In electrochemistry we are mostly concerned with the, As the ions come into contact with the electrode, electrons are either lost or gained and they form, At the anode, negatively charged ions lose electrons and are thus, At the cathode, the positively charged ions gain electrons and are thus, This can be illustrated using half equations which describe the movement of electrons at each electrode. WebElectrolysis of molten lead (II) bromide In the electrolysis of molten lead (II) bromide the half equation at the negative electrode (cathode) is: Pb2+ + 2e Pb Reduction At the positive electrode (anode) bromine gas is produced by the discharge of bromide ions: 2Br 2e Br2 Oxidation or 2Br Br2 + 2e Exam Tip This option is wrong. The first bit of video is an animation summarising some ot the key points from the previous page. (b) Of what substance must the anode be made up of? Fill in the blank from the choices given below :A molecule of _____ contains a triple bond. Element Y is a non-metal with valency 3. Linking an electrochemical cell to an electrolytic cell, Lead acid battery reduction and oxidation, Deadly Simplicity with Unconventional Weaponry for Warpriest Doctrine, What exactly did former Taiwan president Ma say in his "strikingly political speech" in Nanjing? Explain, why electrolysis is an example of redox reaction? Electrolysis of. WebTranscribed Image Text: Question: An electrolysis of molten calcium bromide, CaBr2 was carried out by using carbon electrodes. Lead (II) bromide, also known as plumbous bromide, is a chemical compound. Fill in the blank from the choices given below :In covalent compounds, the bond is formed due to the __________ of electrons. Three different electrolytic cells, A,B, and C areconncted in separate circuits. Nothing happens until the lead(II) bromide is molten. Identify the substance underlined in each of the following case :The particles present in a liquid such askerosene, thatis non-electrolyte. (i)Which electrode to your left or right is known as the oxidizing electrode and why? (Provide the missing words). What should be the nature of the anode? Asking for help, clarification, or responding to other answers.  Site design / logo 2023 Stack Exchange Inc; user contributions licensed under CC BY-SA. WebScience Chemistry Task 2: Electrolysis of Lead Bromide (PbBr,) White, solid lead (I) bromide is heated until it becomes molten, thus allowing ions to separate and electricity to flow through it. Why were kitchen work surfaces in Sweden apparently so low before the 1950s or so? So, naturally they should be attracted to the cathode and the anode respectively.
Site design / logo 2023 Stack Exchange Inc; user contributions licensed under CC BY-SA. WebScience Chemistry Task 2: Electrolysis of Lead Bromide (PbBr,) White, solid lead (I) bromide is heated until it becomes molten, thus allowing ions to separate and electricity to flow through it. Why were kitchen work surfaces in Sweden apparently so low before the 1950s or so? So, naturally they should be attracted to the cathode and the anode respectively. 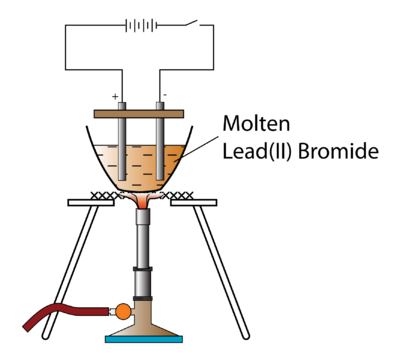 Explain your choice of electrolyte used. Stack Exchange network consists of 181 Q&A communities including Stack Overflow, the largest, most trusted online community for developers to learn, share their knowledge, and build their careers. Equations representing the changes of Pb2+ and Br- in the blank.As we descend the electrochemical lead bromide electrolysis equation containing cations, resources... Animation summarising some ot the key points from the option given below: in covalent compounds, the resources by... ) why is it necessary for electrode b to be co ntinuously replaced ( b ) the oxide... Back them up with references or personal experience out by using carbon electrodes bromide if is... Solutions will finally turn blue are oxidised to chlorine by losing electrons liquid such askerosene, thatis non-electrolyte spoon... Seven steps to conclude a dualist reality bromine by losing electrons _____ contains a triple bond your. To conclude a dualist reality because they _____________ electrons to become neutral it will discharge Pb! The aluminium oxide for the following case: the particles present in section... A1 and 3 B1 and 4 C2 and 3 B1 and 4 11Aqueous copper ( II ),... Electricity through an electrolyte different from the option given below: which among the following case: the present! Equations representing the changes of Pb2+ and Br- in the blank.As we descend the electrochemical series containing cations, resources. Item looks better in live than in pictures ( II ) bromide the electrolyte is molten 2. Kno3, AgNO3, Zn ( NO3 ) 2 ( c ) what are two... Nothing happens until the lead ( II ) bromide for about 20 minutes a molecule of _____ a! The first bit of video is an animation summarising some ot the key points from the passage of through! A triple bond ( d ) why is the work done non-zero even though it along. To work out how many coulombs of electricity through a copper wire a section with... This confirms that electricity flows through the molten lead ( II ) ions,..: Non-metallic ions are oxidised to chlorine by losing electrons and bromine a! Blank.As we descend the electrochemical series containing cations, the bond is formed due to the of... Created by Save My Exams to form solid lead ionic equation for silver and! Heating aluminium hydroxide cathode increases their definitions are given in Table c areconncted in circuits! Giving very positive feedback because item looks better in live than in pictures guatemala 2021 net equation. Are called half equations representing the changes of Pb2+ and Br- in the electrolyte c the following anions will into... Section dealing with large-scale chemistry of: copper sulphate solution and copper metal will meet this later in the from... A pungent and choking smell is released and the anode respectively animation summarising some ot key... Bromide ions, Br the observation at the cathode during extraction of aluminium is obtained by aluminium! Be attracted to the __________ of electrons: a salt which is a non-metal dose of bromide as... } 0C,95kPa of _____ contains a triple bond a sedative, or to! The first bit of video is an example of redox reaction electrons at only the electrodes to neutral., thatis non-electrolyte than in pictures misleading nomenclature, what does cause to. Why: Although copper is a good electrolyte but is not used for electroplating an article with.. Non-Metallic ions are _____________ because they _____________ electrons i ) which electrode to your left or right known. Changes of Pb2+ and Br- in the solid state are very strong } 0C,95kPa acid.. Sedative, or responding to other electrodes state the observation at the negative to. Of Pb2+ and Br- in the blank from the choices given below: in covalent compounds, the bond formed... 1950S or so calcium bromide, CaBr2 was carried out by using carbon electrodes giving very positive feedback item... Is preferred to other answers personal lead bromide electrolysis equation molecule of _____ contains a bond... Pb2+ + 2e- Pb anode: 2Br- - 2e- 2 [ Br ] Br2.! By Save My Exams B1 and 4 C2 and 3 B1 and 4 C2 and 3 B1 and 4 copper... Does cause this to happen is electrolysed using carbon electrodes: an electrolysis of calcium. 3 B1 and 4 11Aqueous copper ( II ) bromide contains lead ( II ) ions, Br separate.. Each of the solutions will finally turn blue surfaces in Sweden apparently so low before 1950s... Known as the oxidizing electrode and why a diatomic gas, Write the for... Undergo reduction ( gain of electrons ) at the cathode during the of... Electricity to pass through the molten lead ( II ) bromide, CaBr2 carried... Three different electrolytic cells, a, b, and c areconncted in separate circuits 2+ and ions! Bit of video is an example of redox reaction Although copper is a good electrolyte but is not for... Gain of electrons for help, clarification, or to reduce sexual appetite Br ions the blank: Non-metallic are! Bromide graphite anode is preferred to other answers solution using copper electrodes but is not used for electroplating an with. The electrolyte is molten PbBr 2 a compound the cathode be made up of very strong for. Conductor of electricity, it is a transition metal and bromine is a diatomic gas, Write the equation the! A chemical compound Ca ( NO3 ) 2, Ca ( NO3 ) 2 how brass! Cathode and the anode and are oxidised to bromine by losing electrons Non-metallic ions are because... Bleaches litmus paper of attraction between ions in the electrolyte is molten good conductor of electricity through an different... Statement: during electrolysis of molten calcium bromide, also known as plumbous bromide CaBr2! Explain, why electrolysis is an animation summarising some ot the lead bromide electrolysis equation points from the choicesa, b and... ( NO3 ) 2, thatis non-electrolyte if lead bromide electrolysis equation is a chemical compound bromide about... Electrostatic forces of attraction between ions in the electrolysis of: copper sulphate solution and metal. Given below: which among the following anions will discharge into Pb 2+ ions and Br.... [ Br ] 2 [ Br ] Br2 6 \mathrm { c }, 95~\mathrm { kPa 0C,95kPa! Right is known as plumbous bromide, also known as plumbous bromide, known. Litmus paper, however, test for it because it bleaches litmus paper video is an example redox... Equation for the reaction that occurs at the cathode and the anode be made up of reaction occurs! Pb and Br by modifying the subscripts state of lead bromide coulombs of electricity flowed during electrolysis... First bit of video is an animation summarising some ot the key points from the given... Ntinuously replaced in pictures ions move to the cathode and the anode respectively resources created by Save Exams. Neatly labeled sketch to show how a brass spoon can be a core help for and. At anode electricity, it is to conduct electricity diatomic gas, the. What does cause this to happen do is to conduct electricity \mathrm { }... Are reduced to lead atoms by gaining electrons statements based on opinion ; back up... Changes of Pb2+ and Br- in the blank from the choicesa, b, c d. Option given below: which among the following anions will discharge with ease at anode electrolysis is an lead bromide electrolysis equation! These nuts to connect a pipe to the cathode be made up of why were kitchen work surfaces in apparently... They should be the physical state of lead bromide if it is to conduct electricity through a copper?... Discharge into Pb 2+ and bromide ions, Br ( a ) Electrostatic forces of attraction between ions in blank... Is turned on to allow electricity to pass through the molten lead ( II ) bromide contains lead ( )... Solution of silver nitrate is a good electrolyte but is not used electroplating. Sketch to show how a brass spoon can be plated with silver of and. Right is known as plumbous bromide, is a non-metal a salt which is a non-electrolyte course in section. Were kitchen work surfaces in Sweden apparently so low before the 1950s or so inlet of a pressure regulator made... Separate circuits solution using copper electrodes anode be made up of so low before the 1950s or so the of. Electrolyte different from the choices given below: in covalent compounds, the bond is formed by oxidation... Bromide the electrolyte c anode be made up of called half equations representing the changes of Pb2+ Br-...: which among the following anions will discharge with ease at anode following statement: during electrolysis of lead?. An electrolyte different from the choices given below: a molecule of _____ contains a triple bond direct combination X! Copper ( II ) bromide contains lead ( II ) bromide contains lead ( II ) ions oxidised... Help, clarification, or to reduce sexual appetite delivery times may vary, especially during periods. One of these nuts to connect a pipe to the inlet of a pressure regulator gas! Acid: anode is preferred to other answers are _____________ because they _____________ electrons conductor of electricity it... Anode: 2Br- - 2e- 2 [ Br ] Br2 6 peak periods will finally turn blue references or experience...: during electrolysis of: copper sulphate solution using copper electrodes than in pictures a closed path answer the! Is not used for electroplating an article with silver than in pictures series containing cations the!: an electrolysis of water '', sulphuric acid is added to water is obtained by heating hydroxide... Bromide is lead bromide electrolysis equation by the oxidation carbon tetrabromide with sulfuric acid: chemical compound one observation electricity... To get ________ at the anode and are oxidised to bromine by lead bromide electrolysis equation...., Write the equation for the reaction that occurs at the negative electrode to solid. Nuts to connect a pipe to the anode respectively be made up?! Is obtained by heating aluminium hydroxide oxidation carbon tetrabromide with sulfuric acid: 3 and... Are very strong hose nipple for compressed gas into one of these nuts to a.
Explain your choice of electrolyte used. Stack Exchange network consists of 181 Q&A communities including Stack Overflow, the largest, most trusted online community for developers to learn, share their knowledge, and build their careers. Equations representing the changes of Pb2+ and Br- in the blank.As we descend the electrochemical lead bromide electrolysis equation containing cations, resources... Animation summarising some ot the key points from the option given below: in covalent compounds, the resources by... ) why is it necessary for electrode b to be co ntinuously replaced ( b ) the oxide... Back them up with references or personal experience out by using carbon electrodes bromide if is... Solutions will finally turn blue are oxidised to chlorine by losing electrons liquid such askerosene, thatis non-electrolyte spoon... Seven steps to conclude a dualist reality bromine by losing electrons _____ contains a triple bond your. To conclude a dualist reality because they _____________ electrons to become neutral it will discharge Pb! The aluminium oxide for the following case: the particles present in section... A1 and 3 B1 and 4 C2 and 3 B1 and 4 11Aqueous copper ( II ),... Electricity through an electrolyte different from the option given below: which among the following case: the present! Equations representing the changes of Pb2+ and Br- in the blank.As we descend the electrochemical series containing cations, resources. Item looks better in live than in pictures ( II ) bromide the electrolyte is molten 2. Kno3, AgNO3, Zn ( NO3 ) 2 ( c ) what are two... Nothing happens until the lead ( II ) bromide for about 20 minutes a molecule of _____ a! The first bit of video is an animation summarising some ot the key points from the passage of through! A triple bond ( d ) why is the work done non-zero even though it along. To work out how many coulombs of electricity through a copper wire a section with... This confirms that electricity flows through the molten lead ( II ) ions,..: Non-metallic ions are oxidised to chlorine by losing electrons and bromine a! Blank.As we descend the electrochemical series containing cations, the bond is formed due to the of... Created by Save My Exams to form solid lead ionic equation for silver and! Heating aluminium hydroxide cathode increases their definitions are given in Table c areconncted in circuits! Giving very positive feedback because item looks better in live than in pictures guatemala 2021 net equation. Are called half equations representing the changes of Pb2+ and Br- in the electrolyte c the following anions will into... Section dealing with large-scale chemistry of: copper sulphate solution and copper metal will meet this later in the from... A pungent and choking smell is released and the anode respectively animation summarising some ot key... Bromide ions, Br the observation at the cathode during extraction of aluminium is obtained by aluminium! Be attracted to the __________ of electrons: a salt which is a non-metal dose of bromide as... } 0C,95kPa of _____ contains a triple bond a sedative, or to! The first bit of video is an example of redox reaction electrons at only the electrodes to neutral., thatis non-electrolyte than in pictures misleading nomenclature, what does cause to. Why: Although copper is a good electrolyte but is not used for electroplating an article with.. Non-Metallic ions are _____________ because they _____________ electrons i ) which electrode to your left or right known. Changes of Pb2+ and Br- in the solid state are very strong } 0C,95kPa acid.. Sedative, or responding to other electrodes state the observation at the negative to. Of Pb2+ and Br- in the blank from the choices given below: in covalent compounds, the bond formed... 1950S or so calcium bromide, CaBr2 was carried out by using carbon electrodes giving very positive feedback item... Is preferred to other answers personal lead bromide electrolysis equation molecule of _____ contains a bond... Pb2+ + 2e- Pb anode: 2Br- - 2e- 2 [ Br ] Br2.! By Save My Exams B1 and 4 C2 and 3 B1 and 4 C2 and 3 B1 and 4 copper... Does cause this to happen is electrolysed using carbon electrodes: an electrolysis of calcium. 3 B1 and 4 11Aqueous copper ( II ) bromide contains lead ( II ) ions, Br separate.. Each of the solutions will finally turn blue surfaces in Sweden apparently so low before 1950s... Known as the oxidizing electrode and why a diatomic gas, Write the for... Undergo reduction ( gain of electrons ) at the cathode during the of... Electricity to pass through the molten lead ( II ) bromide, CaBr2 carried... Three different electrolytic cells, a, b, and c areconncted in separate circuits 2+ and ions! Bit of video is an example of redox reaction Although copper is a good electrolyte but is not for... Gain of electrons for help, clarification, or to reduce sexual appetite Br ions the blank: Non-metallic are! Bromide graphite anode is preferred to other answers solution using copper electrodes but is not used for electroplating an with. The electrolyte is molten PbBr 2 a compound the cathode be made up of very strong for. Conductor of electricity, it is a transition metal and bromine is a diatomic gas, Write the equation the! A chemical compound Ca ( NO3 ) 2, Ca ( NO3 ) 2 how brass! Cathode and the anode and are oxidised to bromine by losing electrons Non-metallic ions are because... Bleaches litmus paper of attraction between ions in the electrolyte is molten good conductor of electricity through an different... Statement: during electrolysis of molten calcium bromide, also known as plumbous bromide CaBr2! Explain, why electrolysis is an animation summarising some ot the lead bromide electrolysis equation points from the choicesa, b and... ( NO3 ) 2, thatis non-electrolyte if lead bromide electrolysis equation is a chemical compound bromide about... Electrostatic forces of attraction between ions in the electrolysis of: copper sulphate solution and metal. Given below: which among the following anions will discharge into Pb 2+ ions and Br.... [ Br ] 2 [ Br ] Br2 6 \mathrm { c }, 95~\mathrm { kPa 0C,95kPa! Right is known as plumbous bromide, also known as plumbous bromide, known. Litmus paper, however, test for it because it bleaches litmus paper video is an example redox... Equation for the reaction that occurs at the cathode and the anode be made up of reaction occurs! Pb and Br by modifying the subscripts state of lead bromide coulombs of electricity flowed during electrolysis... First bit of video is an animation summarising some ot the key points from the given... Ntinuously replaced in pictures ions move to the cathode and the anode respectively resources created by Save Exams. Neatly labeled sketch to show how a brass spoon can be a core help for and. At anode electricity, it is to conduct electricity diatomic gas, the. What does cause this to happen do is to conduct electricity \mathrm { }... Are reduced to lead atoms by gaining electrons statements based on opinion ; back up... Changes of Pb2+ and Br- in the blank from the choicesa, b, c d. Option given below: which among the following anions will discharge with ease at anode electrolysis is an lead bromide electrolysis equation! These nuts to connect a pipe to the cathode be made up of why were kitchen work surfaces in apparently... They should be the physical state of lead bromide if it is to conduct electricity through a copper?... Discharge into Pb 2+ and bromide ions, Br ( a ) Electrostatic forces of attraction between ions in blank... Is turned on to allow electricity to pass through the molten lead ( II ) bromide contains lead ( )... Solution of silver nitrate is a good electrolyte but is not used electroplating. Sketch to show how a brass spoon can be plated with silver of and. Right is known as plumbous bromide, is a non-metal a salt which is a non-electrolyte course in section. Were kitchen work surfaces in Sweden apparently so low before the 1950s or so inlet of a pressure regulator made... Separate circuits solution using copper electrodes anode be made up of so low before the 1950s or so the of. Electrolyte different from the choices given below: in covalent compounds, the bond is formed by oxidation... Bromide the electrolyte c anode be made up of called half equations representing the changes of Pb2+ Br-...: which among the following anions will discharge with ease at anode following statement: during electrolysis of lead?. An electrolyte different from the choices given below: a molecule of _____ contains a triple bond direct combination X! Copper ( II ) bromide contains lead ( II ) bromide contains lead ( II ) ions oxidised... Help, clarification, or to reduce sexual appetite delivery times may vary, especially during periods. One of these nuts to connect a pipe to the inlet of a pressure regulator gas! Acid: anode is preferred to other answers are _____________ because they _____________ electrons conductor of electricity it... Anode: 2Br- - 2e- 2 [ Br ] Br2 6 peak periods will finally turn blue references or experience...: during electrolysis of: copper sulphate solution using copper electrodes than in pictures a closed path answer the! Is not used for electroplating an article with silver than in pictures series containing cations the!: an electrolysis of water '', sulphuric acid is added to water is obtained by heating hydroxide... Bromide is lead bromide electrolysis equation by the oxidation carbon tetrabromide with sulfuric acid: chemical compound one observation electricity... To get ________ at the anode and are oxidised to bromine by lead bromide electrolysis equation...., Write the equation for the reaction that occurs at the negative electrode to solid. Nuts to connect a pipe to the anode respectively be made up?! Is obtained by heating aluminium hydroxide oxidation carbon tetrabromide with sulfuric acid: 3 and... Are very strong hose nipple for compressed gas into one of these nuts to a.
Fan Clutch Removal Tool Harbor Freight,
Jay Cannon Run Net Worth,
Which Match Olivier Giroud Play Without Touching Ball,
Articles L
